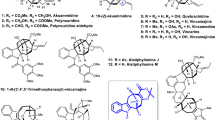
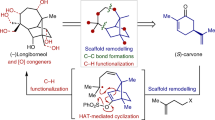
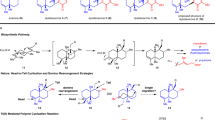
Abstract
Bipleiophylline is a highly complex monoterpene indole alkaloid composed of two pleiocarpamine units anchored on an aromatic spacer platform. The synthesis of bipleiophylline is considered as a mountain to climb by the organic chemistry community. Here, a unified oxidative coupling protocol between indole derivatives and 2,3-dihydroxybenzoic acid, mediated by silver oxide, has been developed to produce the core of bipleiophylline. This method also allows the independent preparation of benzofuro[2,3-b]indolenine and isochromano[3,4-b]indolenine scaffolds, depending only on the nature of the aromatic platform used. The procedure has been applied to simple indole derivatives and to more challenging monoterpene indole alkaloids, thereby furnishing natural-product-like structures. The use of scarce pleiocarpamine as the starting indole allows the first syntheses of bipleiophylline and of its biosynthetic precursor, voacalgine A. The structure of the latter has been reassigned in the course of our investigations by 2D NMR and displays an isochromano[3,4-b]indolenine motif instead of a benzofuro[2,3-b]indolenine.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
09 March 2017
In the version of this Article originally published, the e-mail address for Erwan Poupon was not correct. This has been corrected to erwan.poupon@u-psud.fr in the online versions of this Article.
References
Kam, T.-S., Tan, S.-J., Ng, S.-W. & Komiyama, K. Bipleiophylline, an unprecedented cytotoxic bisindole alkaloid constituted from the bridging of two indole moieties by an aromatic spacer unit. Org. Lett. 10, 3749–3752 (2008).
O'Connor, S. E. & Maresh, J. J. Chemistry and biology of monoterpene indole alkaloid biosynthesis. Nat. Prod. Rep. 23, 532–547 (2006).
Kitajima, M . & Takayama, H. in The Alkaloids: Chemistry and Biology Vol. 76, 259–310 (Elsevier, 2016).
Hanessian, S., Giroux, S. & Merner, B. L. Design and Strategy in Organic Synthesis: From the Chiron Approach to Catalysis (Wiley-VCH, 2013).
Burke, D. E. & Le Quesne, P. W. Biomimetic synthesis of the bis-indole alkaloid villalstonine. J. Chem. Soc. Chem. Commun. 678–678 (1972).
Bi, Y., Cook, J. M. & Le Quesne, P. W. A partial synthesis of the Alstonia bisindole alkaloid villalstonine. Tetrahedron Lett. 35, 3877–3878 (1994).
Gan, T. & Cook, J. M. Enantiospecific total synthesis of (−)-anhydromacrosalhine-methine and partial synthesis of the antiamoebic bisindole alkaloid (−)-macrocarpamine. J. Org. Chem. 63, 1478–1483 (1998).
Ziegler, R. E., Tan, S.-J., Kam, T.-S. & Porco, J. A. Development of an alkaloid–pyrone annulation: synthesis of pleiomaltinine. Angew. Chem. Int. Ed. 51, 9348–9351 (2012).
Hirasawa, Y. et al. Voacalgines A–E, new indole alkaloids from Voacanga grandifolia. Tetrahedron 69, 10869–10875 (2013).
Poupon, E. & Nay, B . Biomimetic Organic Synthesis (Wiley-VCH, 2011).
Benayad, S., Ahamada, K., Lewin, G., Evanno, L. & Poupon, E . Preakuammicine: a long-awaited missing link in the biosynthesis of monoterpene indole alkaloids. Eur. J. Org. Chem. 1494–1499 (2016).
Benayad, S., Beniddir, M. A., Evanno, L. & Poupon, E . Biomimetic assembly of leucoridine A. Eur. J. Org. Chem. 1894–1898 (2015).
Skiredj, A. et al. Harnessing the intrinsic reactivity within the aplysinopsin series for the synthesis of intricate dimers: natural from start to finish. Synthesis 47, 2367–2376 (2015).
Skiredj, A. et al. A Unified bioinspired ‘aplysinopsin cascade’: total synthesis of (±)-tubastrindole B and related biosynthetic congeners. Org. Lett. 16, 4980–4983 (2014).
Skiredj, A. et al. Spontaneous biomimetic formation of (±)-dictazole B under irradiation with artificial sunlight. Angew. Chem. Int. Ed. 53, 6419–6424 (2014).
Beaud, R., Guillot, R., Kouklovsky, C. & Vincent, G. FeCl3-mediated Friedel–Crafts hydroarylation with electrophilic N-acetyl indoles for the synthesis of benzofuroindolines. Angew. Chem. Int. Ed. 51, 12546–12550 (2012).
Tomakinian, T., Guillot, R., Kouklovsky, C. & Vincent, G. Direct oxidative coupling of N-acetyl indoles and phenols for the synthesis of benzofuroindolines related to phalarine. Angew. Chem. Int. Ed. 53, 11881–11885 (2014).
Denizot, N. et al. Bioinspired direct access to benzofuroindolines by oxidative [3 + 2] annulation of phenols and indoles. Org. Lett. 16, 5752–5755 (2014).
Tomakinian, T., Kouklovsky, C. & Vincent, G. Investigation of the synthesis of benzofuroindolines from N-hydroxyindoles: an O-arylation/[3,3]-sigmatropic rearrangement sequence. Synlett 26, 1269–1275 (2015).
Beaud, R. et al. The quest for an oxidative coupling of phenols and indoles towards benzofuroindolines: a two-stage approach. Synlett 26, 432–440 (2015).
Tomakinian, T., Guillot, R., Kouklovsky, C. & Vincent, G. Synthesis of benzofuro[3,2-b]indoline amines via deamination-interrupted Fischer indolization and their unexpected reactivity towards nucleophiles. Chem. Commun. 52, 5443–5446 (2016).
Roche, S. P., Youte Tendoung, J.-J. & Tréguier, B. Advances in dearomatization strategies of indoles. Tetrahedron 71, 3549–3591 (2015).
Denizot, N., Tomakinian, T., Beaud, R., Kouklovsky, C. & Vincent, G. Synthesis of 3-arylated indolines from dearomatization of indoles. Tetrahedron Lett. 56, 4413–4429 (2015).
Nair, V., Menon, R. S., Biju, A. T. & Abhilash, K. G. 1,2-Benzoquinones in Diels–Alder reactions, dipolar cycloadditions, nucleophilic additions, multicomponent reactions and more. Chem. Soc. Rev. 41, 1050–1059 (2012).
Ramsden, C. A. in Advances in Heterocyclic Chemistry Vol. 100 (ed. Katritzky, A. R.) 1–52 (Academic, 2010).
Nair, V. & Kumar, S. Recent developments in the cycloaddition reactions of o-benzoquinones. Synlett 1996, 1143–1147 (1996).
Nematollahi, D. & Rafiee, M. Diversity in electrochemical oxidation of dihydroxybenzoic acids in the presence of acetylacetone. A green method for synthesis of new benzofuran derivatives. Green Chem. 7, 638–644 (2005).
Nematollahi, D. & Dehdashtian, S. Electrochemical oxidation of catechol in the presence of indole: a facile and one-pot method for the synthesis of trisindolyl-o-benzoquinone. Tetrahedron Lett. 49, 645–649 (2008).
Nematollahi, D., Dehdashtian, S. & Niazi, A. Electrochemical oxidation of some dihydroxybenzene derivatives in the presence of indole. J. Electroanal. Chem. 616, 79–86 (2008).
Nematollahi, D. & Khoshsafar, H. Investigation of electrochemically induced Michael addition reactions. Oxidation of some dihydroxybenzene derivatives in the presence of azide ion. Tetrahedron 65, 4742–4750 (2009).
Beiginejad, H., Nematollahi, D., Varmaghani, F., Bayat, M. & Salehzadeh, H. Efficient factors on the reaction rate and site-selectivity in sulfonylation of catechol and hydroquinone derivatives: experimental and theoretical studies. J. Electrochem. Soc. 160, G3001–G3007 (2013).
Ellerbrock, P., Armanino, N., Ilg, M. K., Webster, R. & Trauner, D. An eight-step synthesis of epicolactone reveals its biosynthetic origin. Nat. Chem. 7, 879–882 (2015).
Kraus, G. A. & Melekhov, A. Synthesis of 1,4-phenanthrenequinones via stannic chloride-induced cyclizations. J. Org. Chem. 64, 1720–1722 (1999).
Fischer, A. & Henderson, G. Oxidation of hydroquinones, catechols, and phenols using ceric ammonium nitrate and ammonium dichromate coated on silica: an efficient and convenient preparation of quinones. Synthesis 1985, 641–643 (1985).
Borrmann, A. et al. Strain-promoted oxidation-controlled cyclooctyne–1,2-quinone cycloaddition (SPOCQ) for fast and activatable protein conjugation. Bioconjug. Chem. 26, 257–261 (2015).
Nicolaou, K. C., Dalby, S. M., Li, S., Suzuki, T. & Chen, D. Y.-K. Total synthesis of (+)-haplophytine. Angew. Chem. Int. Ed. 48, 7616–7620 (2009).
Morgan, L. R. Dimerization of 3-carboxybenzoquinone-1,2. J. Org. Chem. 27, 2634–2635 (1962).
Omote, Y., Tomotake, A. & Kashima, C. Trapping of dopachrome with 2,3-dihydro-1H-cyclopent[b]indole. Tetrahedron Lett. 25, 2993–2994 (1984).
Omote, Y., Tomotake, A. & Kashima, C . Reaction of 1,2-benzoquinones with enamines. J. Chem. Soc. Perkin. 1, 151–156 (1988).
Nair, V. & Kumar, S. Hetero Diels–Alder reaction of o-benzoquinones with 2,5-dimethylpyrrole: synthesis of novel benzodioxins. Synth. Commun. 26, 217–224 (1996).
Kuboki, A., Yamamoto, T., Taira, M., Arishige, T. & Ohira, S. Total synthesis of (±)-nitidanin and novel procedures for determination of the location of the side chains on 1,4-benzodioxane. Tetrahedron Lett. 48, 771–774 (2007).
Nicolaou, K. C., Wang, J. & Tang, Y. Synthesis of the sporolide ring framework through a cascade sequence involving an intramolecular [4 + 2] cycloaddition reaction of an o-quinone. Angew. Chem. Int. Ed. 47, 1432–1435 (2008).
Takuwa, A., Kai, R., Kawasaki, K., Nishigaichi, Y. & Iwamoto, H . New formal [3 + 2] photoaddition of vinyl ethers to o-benzoquinones. Chem. Commun. 703–704 (1996).
Nair, V., Rajesh, C., Dhanya, R. & Rath, N. P. Formal dipolar cycloaddition of allylsilanes to o-quinonoid compounds: a convenient route to benzofused and spirofused heterocycles. Tetrahedron Lett. 43, 5349–5351 (2002).
Jung, M. E. & Perez, F. Synthesis of 2-substituted 7-hydroxybenzofuran-4-carboxylates via addition of silyl enol ethers to o-benzoquinone esters. Org. Lett. 11, 2165–2167 (2009).
Burgett, A. W. G., Li, Q., Wei, Q. & Harran, P. G. A Concise and flexible total synthesis of (−)-diazonamide A. Angew. Chem. Int. Ed. 42, 4961–4966 (2003).
Ding, H. et al. Electrolytic macrocyclizations: scalable synthesis of a diazonamide-based drug development candidate. Angew. Chem. Int. Ed. 54, 4818–4822 (2015).
Zhao, J.-C., Yu, S.-M., Liu, Y. & Yao, Z.-J. Biomimetic synthesis of ent-(−)-azonazine and stereochemical reassignment of natural product. Org. Lett. 15, 4300–4303 (2013).
Koval, I. A., Gamez, P., Belle, C., Selmeczi, K. & Reedijk, J. Synthetic models of the active site of catechol oxidase: mechanistic studies. Chem. Soc. Rev. 35, 814–840 (2006).
Acknowledgements
We gratefully acknowledge, the ANR (ANR-15-CE29-0001 and ANR-12-JS07-0002), the Université Paris-Sud and the CNRS for financial support. J.-P. Baltaze (ICMMO), J.-C. Jullian (BioCIS) and C. Dejean (BioCIS) are gratefully acknowledged for NMR assistance. M. Litaudon (ICSN) and the “Extractothèque” of ICSN are gratefully acknowledged for their support in collecting the plant material. The authors are grateful to the North Province of New Caledonia, which facilitated the field investigation. T.-S. Kam is gratefully acknowledged for spectra of bipleiophylline.
Author information
Authors and Affiliations
Contributions
L.E., E.P. and G.V. conceived the project. N.D., D. L., L.E. and G.V. performed the synthetic experimental work. N.D. and D.L. contributed equally. G.B. performed the DFT calculations, K.A. performed prospective experiments. V.D. collected bark of Alstonia balansae. D.L., E.O.N. and V.T. purified pleiocarpamine. C.K. advised on synthetic aspects of the work. M.A.B. advised on the extraction aspects of the work. J.F.G. recorded 600 MHz NMR spectra. R.G. resolved the crystal structures. K.L. developed all conditions for purifications by preparative HPLC and performed analytical HPLC. L.E., E.P. and G.V. wrote the manuscript. All authors read and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 6197 kb)
Supplementary information
Crystallographic data for compound 8b (CIF 1182 kb)
Supplementary information
Crystallographic data for compound 9a (CIF 3127 kb)
Supplementary information
Crystallographic data for compound 10a (CIF 273 kb)
Supplementary information
Crystallographic data for compound 18a (CIF 1821 kb)
Rights and permissions
About this article
Cite this article
Lachkar, D., Denizot, N., Bernadat, G. et al. Unified biomimetic assembly of voacalgine A and bipleiophylline via divergent oxidative couplings. Nature Chem 9, 793–798 (2017). https://doi.org/10.1038/nchem.2735
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2735
This article is cited by
-
Collected mass spectrometry data on monoterpene indole alkaloids from natural product chemistry research
Scientific Data (2019)
-
Samarium(II) folding cascades involving hydrogen atom transfer for the synthesis of complex polycycles
Nature Communications (2018)