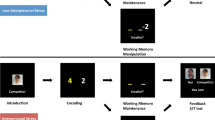
Abstract
More than half of the world’s population now lives in cities, making the creation of a healthy urban environment a major policy priority1. Cities have both health risks and benefits1, but mental health is negatively affected: mood and anxiety disorders are more prevalent in city dwellers2 and the incidence of schizophrenia is strongly increased in people born and raised in cities3,4,5,6. Although these findings have been widely attributed to the urban social environment2,3,7,8, the neural processes that could mediate such associations are unknown. Here we show, using functional magnetic resonance imaging in three independent experiments, that urban upbringing and city living have dissociable impacts on social evaluative stress processing in humans. Current city living was associated with increased amygdala activity, whereas urban upbringing affected the perigenual anterior cingulate cortex, a key region for regulation of amygdala activity, negative affect9 and stress10. These findings were regionally and behaviourally specific, as no other brain structures were affected and no urbanicity effect was seen during control experiments invoking cognitive processing without stress. Our results identify distinct neural mechanisms for an established environmental risk factor, link the urban environment for the first time to social stress processing, suggest that brain regions differ in vulnerability to this risk factor across the lifespan, and indicate that experimental interrogation of epidemiological associations is a promising strategy in social neuroscience.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Dye, C. Health and urban living. Science 319, 766–769 (2008)
Peen, J., Schoevers, R. A., Beekman, A. T. & Dekker, J. The current status of urban-rural differences in psychiatric disorders. Acta Psychiatr. Scand. 121, 84–93 (2010)
Krabbendam, L. & van Os, J. Schizophrenia and urbanicity: a major environmental influence–conditional on genetic risk. Schizophr. Bull. 31, 795–799 (2005)
Mortensen, P. B. et al. Effects of family history and place and season of birth on the risk of schizophrenia. N. Engl. J. Med. 340, 603–608 (1999)
Pedersen, C. B. & Mortensen, P. B. Evidence of a dose-response relationship between urbanicity during upbringing and schizophrenia risk. Arch. Gen. Psychiatry 58, 1039–1046 (2001)
van Os, J., Pedersen, C. B. & Mortensen, P. B. Confirmation of synergy between urbanicity and familial liability in the causation of psychosis. Am. J. Psychiatry 161, 2312–2314 (2004)
van Os, J., Kenis, G. & Rutten, B. P. The environment and schizophrenia. Nature 468, 203–212 (2010)
Selten, J. P. & Cantor-Graae, E. Social defeat: risk factor for schizophrenia? Br. J. Psychiatry 187, 101–102 (2005)
Pezawas, L. et al. 5-HTTLPR polymorphism impacts human cingulate–amygdala interactions: a genetic susceptibility mechanism for depression. Nature Neurosci. 8, 828–834 (2005)
Diorio, D., Viau, V. & Meaney, M. J. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J. Neurosci. 13, 3839–3847 (1993)
Weinberger, D. R. Implications of normal brain development for the pathogenesis of schizophrenia. Arch. Gen. Psychiatry 44, 660–669 (1987)
Meyer-Lindenberg, A. From maps to mechanisms through neuroimaging of schizophrenia. Nature 468, 194–202 (2010)
Dickerson, S. S. & Kemeny, M. E. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol. Bull. 130, 355–391 (2004)
Dedovic, K. et al. The Montreal Imaging Stress Task: using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. J. Psychiatry Neurosci. 30, 319–325 (2005)
Lederbogen, F. et al. Salivary cortisol in a middle-aged community sample: results from 990 men and women of the KORA-F3 Augsburg study. Eur. J. Endocrinol. 163, 443–451 (2010)
Pruessner, J. C. et al. Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol. Psychiatry 63, 234–240 (2008)
Esslinger, C. et al. Neural mechanisms of a genome-wide supported psychosis variant. Science 324, 605 (2009)
LeDoux, J. E. Emotion circuits in the brain. Annu. Rev. Neurosci. 23, 155–184 (2000)
Meyer-Lindenberg, A. et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc. Natl Acad. Sci. USA 103, 6269–6274 (2006)
Herman, J. P., Ostrander, M. M., Mueller, N. K. & Figueiredo, H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog. Neuropsychopharmacol. Biol. Psychiatry 29, 1201–1213 (2005)
Wright, I. C. et al. Meta-analysis of regional brain volumes in schizophrenia. Am. J. Psychiatry 157, 16–25 (2000)
Vidal, C. N. et al. Dynamically spreading frontal and cingulate deficits mapped in adolescents with schizophrenia. Arch. Gen. Psychiatry 63, 25–34 (2006)
Rasetti, R. et al. Evidence that altered amygdala activity in schizophrenia is related to clinical state and not genetic risk. Am. J. Psychiatry 166, 216–225 (2009)
Poeggel, G. et al. Juvenile emotional experience alters synaptic composition in the rodent cortex, hippocampus, and lateral amygdala. Proc. Natl Acad. Sci. USA 100, 16137–16142 (2003)
Bickart, K. C., Wright, C. I., Dautoff, R. J., Dickerson, B. C. & Barrett, L. F. Amygdala volume and social network size in humans. Nature Neurosci. 14, 163–164 (2011)
Spinelli, S. et al. Early-life stress induces long-term morphologic changes in primate brain. Arch. Gen. Psychiatry 66, 658–665 (2009)
Cohen, R. A. et al. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol. Psychiatry 59, 975–982 (2006)
Entringer, S., Kumsta, R., Hellhammer, D. H., Wadhwa, P. D. & Wust, S. Prenatal exposure to maternal psychosocial stress and HPA axis regulation in young adults. Horm. Behav. 55, 292–298 (2009)
Zink, C. F. et al. Know your place: neural processing of social hierarchy in humans. Neuron 58, 273–283 (2008)
Zink, C. F., Stein, J. L., Kempf, L., Hakimi, S. & Meyer-Lindenberg, A. Vasopressin modulates medial prefrontal cortex–amygdala circuitry during emotion processing in humans. J. Neurosci. 30, 7017–7022 (2010)
Acknowledgements
We thank D. Gass and C. Niemeyer for technical assistance. We also thank C. Sauer, O. Grimm, M. Plichta and A. Schäfer for support on data analyses. The research leading to these results has received funding from the European Community’s Seventh Framework Programme under grant agreement No. HEALTH-F2-2010-241909 (Project EU-GEI), German Research Foundation (Deutsche Forschungsgemeinschaft SFB 636-B7) and Federal Ministry of Education and Research (MooDS) to A.M.L. EU-GEI is an acronym for the project “European network of National Schizophrenia Networks Studying Gene–Environment Interactions”. F.S. is a member of the International Research Training Group “Psychoneuroendocrinology of Stress”, Institute of Psychobiology, University of Trier, Germany, granted by the German Research Foundation.
Author information
Authors and Affiliations
Contributions
F.L., P.K and L.H. designed and performed experiments, analysed data and wrote the paper; F.S., P.S. and S.W. designed and performed experiments, analysed data and reviewed the manuscript; H.T. analysed data and reviewed the manuscript; M.D. and M.R. designed experiments and reviewed the manuscript; J.C.P. developed the MIST paradigm and reviewed the manuscript. A.M.-L. obtained funding, designed the study and experiments and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Methods, with additional references, Supplementary Tables 1-3 and Supplementary Figures 1-3 with legends. (PDF 766 kb)
PowerPoint slides
Rights and permissions
About this article
Cite this article
Lederbogen, F., Kirsch, P., Haddad, L. et al. City living and urban upbringing affect neural social stress processing in humans. Nature 474, 498–501 (2011). https://doi.org/10.1038/nature10190
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10190
This article is cited by
-
Happiness and air quality: microdata analysis in Indonesia
Journal of Health, Population and Nutrition (2024)
-
Abnormal functional neurocircuitry underpinning emotional processing in fibromyalgia
European Archives of Psychiatry and Clinical Neuroscience (2024)
-
Effects of green and urban environment exposure during classroom breaks in a video-based setting
Journal of Outdoor and Environmental Education (2024)
-
Life course rural/urban place of residence, depressive symptoms and cognitive impairment among older adults: findings from the Longitudinal Aging Study in India
BMC Psychiatry (2023)
-
Rising trends in the burden of migraine and tension-type headache among adolescents and young adults globally, 1990 to 2019
The Journal of Headache and Pain (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.