Abstract
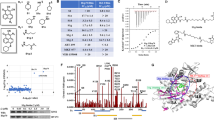
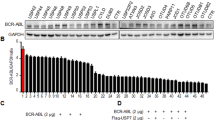
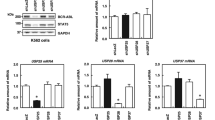
The Bcr-Abl fusion gene encodes for the p210Bcr-Abl or p185Bcr-Abl tyrosine kinase (TK) implicated in the pathogenesis of chronic myelogenous leukemia (CML) or acute lymphoblastic leukemia, respectively. Because Bcr-Abl TK is chaperoned by Hsp90 (90 kDa heat-shock protein), we investigated the effects of novobiocin (NB), an Hsp90 C-terminal inhibitor, on the viability of the Bcr-Abl-positive human leukemia cells HL-60/Bcr-Abl and K562, the expression of Bcr-Abl protein and the interaction between Hsp90 and Bcr-Abl TK. Present studies demonstrate that NB is a potent inhibitor of the growth of Bcr-Abl-positive human leukemia cells. NB induces cytosolic accumulation of cytochrome c and activation of caspase-9 and caspase-3, triggering apoptosis of HL-60/Bcr-Abl and K562 cells. Treatment of cell lines with NB disrupts Bcr-Abl /Hsp90 and Bcr-Abl /Hsp70 interactions, resulting in a decreased amount of intracellular Bcr-Abl protein levels. Co-treatment with the proteasome inhibitor N-acetyl leucyl-leucyl norlucinal increases NB-mediated accumulation of Bcr-Abl in the detergent-insoluble cellular fraction, which demonstrates that NB promotes proteasomal degradation of Bcr-Abl. Moreover, both imatinib-resistant K562/G01 and primary CML CD34+ cells are sensitive to NB.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Blagosklonny MV, Toretsky J, Neckers L . Geldanamycin selectively destabilizes and conformationally alters mutated p53. Oncogene 1995; 11: 933–939.
Chavany C, Mimnaugh E, Miller P, Bitton R, Nguyen P, Trepel J et al. p185erbB2 binds to GRP94 in vivo. Dissociation of the p185erbB2/GRP94 heterocomplex by benzoquinone ansamycins precedes depletion of p185erbB2. J Biol Chem 1996; 271: 4974–4977.
Stepanova L, Leng X, Parker SB, Harper JW . Mammalian p50Cdc37 is a protein kinase-targeting subunit of Hsp90 that binds and stabilizes Cdk4. Genes Dev 1996; 10: 1491–1502.
Scheibel T, Neuhofen S, Weikl T, Mayr C, Reinstein J, Vogel PD et al. ATP-binding properties of human Hsp90. J Biol Chem 1997; 272: 18608–18613.
Scheibel T, Weikl T, Buchner J . Two chaperone sites in Hsp90 differing in substrate specificity and ATP dependence. Proc Natl Acad Sci USA 1998; 95: 1495–1499.
Grenert JP, Sullivan WP, Fadden P, Haystead TAJ, Clark J, Mimnaugh E et al. The amino-terminal domain of heat shock protein 90 (hsp90) that binds geldanamycin is an ATP/ADP switch domain that regulates hsp90 conformation. J Biol Chem 1997; 272: 23843–23850.
Grenert JP, Johnson BD, Toft DO . The importance of ATP binding and hydrolysis by hsp90 in formation and function of protein heterocomplexes. J Biol Chem 1999; 274: 17525–17533.
Marcu MG, Schulte TW, Neckers L . Novobiocin and related coumarins and depletion of heat shock protein 90-dependent signaling proteins. J Natl Cancer Inst 2000; 92: 242–248.
Allan RK, Mok D, Ward BK, Ratajczak T . Modulation of chaperone function and cochaperone interaction by novobiocin in the C-terminal domain of Hsp90: evidence that coumarin antibiotics disrupt Hsp90 dimerization. J Biol Chem 2006; 281: 7161–7171.
Faderl S, Talpaz M, Estrov Z, Kantarjian HM . Chronic myelogenous leukemia. Biology and therapy. Ann Intern Med 1999; 131: 207–219.
Bagatell R, Whitesell L . Altered Hsp90 function in cancer: a unique therapeutic opportunity. Mol Cancer Ther 2004; 3: 1021–1030.
Nimmanapalli R, O’Bryan E, Bhalla K . Geldanamycin and its analogue 17-allylamino-17-demethoxygeldanamycin lowers Bcr-Abl levels and induces apoptosis and differentiation of Bcr-Abl-positive human leukemic blasts. Cancer Res 2001; 61: 1799–1804.
Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science 2001; 293: 876–880.
von Bubnoff N, Schneller F, Peschel C, Duyster J . BCR-ABL gene mutations in relation to clinical resistance of Philadelphia-chromosome-positive leukaemia to STI571: a prospective study. Lancet 2002; 359: 487–491.
Qi J, Peng H, Gu ZL, Liang ZQ, Yang CZ . Establishment of an imatinib resistant cell line K562/G01 and its characterization. Chin J Hematol 2004; 25: 337–341.
Bhatia R, McGlave PB, Miller JS, Wissink S, Lin WN, Verfaillie CM . A clinical lysuitable ex vivo expansion culture system for LTC-IC and CFC using stroma-conditioned medium. Exp Hematol 1997; 25: 980–991.
Bhatia R, McGlave PB, Dewald GW, Blazar BR, Verfaillie CM . Abnormal function of the bone marrow microenvironment in chronic myelogenous leukemia: role of malignant stromal macrophages. Blood 1995; 85: 3636–3645.
Jow GM, Chou CJ, Chen BF, Tsai JH . Beauvericin induces cytotoxic effects in human acute lymphoblastic leukemia cells through cytochrome c release, caspase 3 activation: the causative role of calcium. Cancer Lett 2004; 216: 165–173.
Wu LX, Xu JH, Huang XW, Zhang KZ, Wen CX, Chen YZ . Down-regulation of p210(bcr/abl) by curcumin involves disrupting molecular chaperone functions of Hsp90. Acta Pharmacol Sin 2006; 27: 694–699.
Perkins CL, Fang G, Kim CN, Bhalla KN . The role of Apaf-1, caspase-9, and Bid proteins in etoposide-or paclitaxel-induced mitochondrial events during apoptosis. Cancer Res 2000; 60: 1645–1653.
King MA . Detection of dead cells and measurement of cell killing by flow cytometry. J Immunol Methods 2000; 243: 155–166.
Amarante-Mendes G, Kim C, Liu L, Huang Y, Perkins C, Green D et al. Bcr-Abl exerts its antiapoptotic effect against diverse apoptotic stimuli through blockage of mitochondrial release of cytochrome c and activation of caspase-3. Blood 1998; 91: 1700–1705.
Guo F, Rocha K, Bali P, Pranpat M, Fiskus W, Boyapalle S et al. Abrogation of heat shock protein 70 induction as a strategy to increase antileukemia activity of heat shock protein 90 inhibitor 17-allylamino-demethoxy geldanamycin. Cancer Res 2005; 65: 10536–10544.
Guo F, Sigua C, Bali P, George P, Fiskus W, Scuto A et al. Mechanistic role of heat shock protein 70 in Bcr-Abl–mediated resistance to apoptosis in human acute leukemia cells. Blood 2005; 105: 1246–1255.
Johnson BD, Schumacher RJ, Ross ED, Toft DO . Hop modulates Hsp70/Hsp90 interactions in protein folding. J Biol Chem 1998; 273: 3679–3686.
Chen S, Prapapanich V, Rimerman RA, Honore B, Smith DF . Interactions of p60, a mediator of progesterone receptor assembly, with heat shock proteins hsp90 and hsp70. Mol Endocrinol 1996; 10: 682–693.
Drusano GL, Townsend RJ, Walsh TJ, Forrest A, Antal EJ, Standiford HC . Steady-state serum pharmacokinetics of novobiocin and rifampin alone and in combination. Antimicrob Agents Chemother 1986; 30: 42–45.
Burlison JA, Neckers L, Smith AB, Maxwell A, Blagg BSJ . Novobiocin: redesigning a DNA gyrase inhibitor for selective inhibition of Hsp90. J Am Chem Soc 2006; 128: 15529–15536.
Acknowledgements
We thank Dr Kapil Bhalla for kindly providing the HL-60/Bcr-Abl cells and MD Xinjian Lin (University of California, San Diego) for acquiring and eventually distributing the HL-60/Bcr-Abl cells to us. We thank Professor Chunzheng Yang (Institute of Hematology, Chinese Academy of Medical Sciences and Peking Union Medical College, Tianjin, China) for kindly providing the K562/G01 cell line. We also gratefully acknowledge the support of this project by the National Natural Science Foundation of China (30171158 and 30472187) and the Natural Science Foundation of the Fujian Province of China (C992001 and C0610025). This study was supported by grants from the National Natural Science Foundation of China (30171158 and 30472187) and the Natural Science Foundation of Fujian Province of China (C992001 and C0610025).
Author information
Authors and Affiliations
Corresponding authors
Additional information
The authors have retracted this article. An investigation by Fujian Medical University identified multiple errors in the assembly of the figures, most notably in the duplication of loading controls. All authors agree with this retraction.
About this article
Cite this article
Wu, L., Xu, J., Zhang, K. et al. RETRACTED ARTICLE: Disruption of the Bcr-Abl/Hsp90 protein complex: a possible mechanism to inhibit Bcr-Abl-positive human leukemic blasts by novobiocin. Leukemia 22, 1402–1409 (2008). https://doi.org/10.1038/leu.2008.89
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2008.89
Keywords
This article is cited by
-
Retraction Note: Disruption of the Bcr-Abl/Hsp90 protein complex: a possible mechanism to inhibit Bcr-Abl-positive human leukemic blasts by novobiocin
Leukemia (2020)
-
Non ABL-directed inhibitors as alternative treatment strategies for chronic myeloid leukemia
Molecular Cancer (2018)
-
Effect of novobiocin on the viability of human gingival fibroblasts (HGF-1)
BMC Pharmacology and Toxicology (2014)
-
Curcumin derivative C817 inhibits proliferation of imatinib-resistant chronic myeloid leukemia cells with wild-type or mutant Bcr-Abl in vitro
Acta Pharmacologica Sinica (2014)
-
HSP90 inhibition results in apoptosis of Philadelphia acute lymphoblastic leukaemia cells: an attractive prospect of new targeted agents
Journal of Cancer Research and Clinical Oncology (2012)