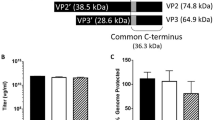
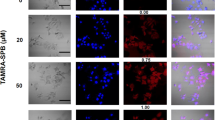
Abstract
Human adenoviruses have a great potential as anticancer agents. One strategy to improve their tumor-cell specificity and anti-tumor efficacy is to include tumor-specific targeting ligands in the viral capsid. This can be achieved by fusion of polypeptide-targeting ligands with the minor capsid protein IX. Previous research suggested that protein IX-mediated targeting is limited by inefficient release of protein IX-fused ligands from their cognate receptors in the endosome. This thwarts endosomal escape of the virus particles. Here we describe that the targeted transduction of tumor cells is augmented by a cathepsin-cleavage site between the protein IX anchor and the HER2/neu-binding ZH Affibody molecule as ligand. The cathepsin-cleavage site did not interfere with virus production and incorporation of the Affibody molecules in the virus capsid. Virus particles harboring the cleavable protein IX–ligand fusion in their capsid transduced the HER2/neu-positive SKOV-3 ovarian carcinoma cells with increased efficiency in monolayer cultures, three-dimensional spheroid cultures and in SKOV-3 tumors grown on the chorioallantoic membrane of embryonated chicken eggs. These data show that inclusion of a cathepsin-cleavage sequence between protein IX and a high-affinity targeting ligand enhances targeted transduction. This modification further augments the applicability of protein IX as an anchor for coupling tumor-targeting ligands.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
de Vrij J, Willemsen RA, Lindholm L, Hoeben RC, Bangma CH, Barber C et al. Adenovirus-derived vectors for prostate cancer gene therapy. Hum Gene Ther 2010; 21: 795–805.
Li D, Duan L, Freimuth P, O’Malley Jr BW . Variability of adenovirus receptor density influences gene transfer efficiency and therapeutic response in head and neck cancer. Clin Cancer Res 1999; 5: 4175–4181.
Hemmi S, Geertsen R, Mezzacasa A, Peter I, Dummer R . The presence of human coxsackievirus and adenovirus receptor is associated with efficient adenovirus-mediated transgene expression in human melanoma cell cultures. Hum Gene Ther 1998; 9: 2363–2373.
Bachtarzi H, Stevenson M, Fisher K . Cancer gene therapy with targeted adenoviruses. Expert Opin Drug Deliv 2008; 5: 1231–1240.
Dmitriev IP, Kashentseva EA, Curiel DT . Engineering of adenovirus vectors containing heterologous peptide sequences in the C terminus of capsid protein IX. J Virol 2002; 76: 6893–6899.
Vellinga J, Rabelink MJ, Cramer SJ, van den Wollenberg DJ, Van der Meulen H, Leppard KN et al. Spacers increase the accessibility of peptide ligands linked to the carboxyl terminus of adenovirus minor capsid protein IX. J Virol 2004; 78: 3470–3479.
Vellinga J, van den Wollenberg DJ, van der Heijdt S, Rabelink MJ, Hoeben RC . The coiled-coil domain of the adenovirus type 5 protein IX is dispensable for capsid incorporation and thermostability. J Virol 2005; 79: 3206–3210.
Vellinga J, de Vrij J, Myhre S, Uil T, Martineau P, Lindholm L et al. Efficient incorporation of a functional hyper-stable single-chain antibody fragment protein-IX fusion in the adenovirus capsid. Gene Therapy 2007; 14: 664–670.
de Vrij J, Uil TG, van den Hengel SK, Cramer SJ, Koppers-Lalic D, Verweij MC et al. Adenovirus targeting to HLA-A1/MAGE-A1-positive tumor cells by fusing a single-chain T-cell receptor with minor capsid protein IX. Gene Therapy 2008; 15: 978–989.
Tang Y, Wu H, Ugai H, Matthews QL, Curiel DT . Derivation of a triple mosaic adenovirus for cancer gene therapy. PLoS One 2009; 4: e8526.
Campos SK, Barry MA . Comparison of adenovirus fiber, protein IX, and hexon capsomeres as scaffolds for vector purification and cell targeting. Virology 2006; 349: 453–462.
Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 1997; 275: 1320–1323.
Nemerow GR, Stewart PL . Role of alpha(v) integrins in adenovirus cell entry and gene delivery. Microbiol Mol Biol Rev 1999; 63: 725–734.
Wickham TJ, Mathias P, Cheresh DA, Nemerow GR . Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell 1993; 73: 309–319.
Sussenbach JS . Early events in the infection process of adenovirus type 5 in HeLa cells. Virology 1967; 33: 567–574.
Greber UF, Willetts M, Webster P, Helenius A . Stepwise dismantling of adenovirus 2 during entry into cells. Cell 1993; 75: 477–486.
Kirschke H, Barrett AJ, Rawlings ND . Proteinases 1: lysosomal cysteine proteinases. Protein Profile 1995; 2: 1581–1643.
Wikman M, Steffen AC, Gunneriusson E, Tolmachev V, Adams GP, Carlsson J et al. Selection and characterization of HER2/neu-binding Affibody ligands. Protein Eng Des Sel 2004; 17: 455–462.
Wang SC, Hung MC . HER2 overexpression and cancer targeting. Semin Oncol 2001; 28: 115–124.
Magnusson MK, Henning P, Myhre S, Wikman M, Uil TG, Friedman M et al. Adenovirus 5 vector genetically re-targeted by an Affibody molecule with specificity for tumor antigen HER2/neu. Cancer Gene Ther 2007; 14: 468–479.
Ono HA, Le LP, Davydova JG, Gavrikova T, Yamamoto M . Noninvasive visualization of adenovirus replication with a fluorescent reporter in the E3 region. Cancer Res 2005; 65: 10154–10158.
Corjon S, Wortmann A, Engler T, van Rooijen N, Kochanek S, Kreppel F . Targeting of adenovirus vectors to the LRP receptor family with the high-affinity ligand RAP via combined genetic and chemical modification of the pIX capsomere. Mol Ther 2008; 16: 1813–1824.
Ebert DH, Deussing J, Peters C, Dermody TS . Cathepsin L and cathepsin B mediate reovirus disassembly in murine fibroblast cells. J Biol Chem 2002; 277: 24609–24617.
Schornberg K, Matsuyama S, Kabsch K, Delos S, Bouton A, White J . Role of endosomal cathepsins in entry mediated by the Ebola virus glycoprotein. J Virol 2006; 80: 4174–4178.
Diederich S, Moll M, Klenk HD, Maisner A . The nipah virus fusion protein is cleaved within the endosomal compartment. J Biol Chem 2005; 280: 29899–29903.
DeNardo GL, DeNardo SJ . Evaluation of a cathepsin-cleavable peptide linked radioimmunoconjugate of a panadenocarcinoma MAb, m170, in mice and patients. Cancer Biother Radiopharm 2004; 19: 85–92.
Barrett AJ, Kirschke H . Cathepsin B, cathepsin H, and cathepsin L. Methods Enzymol 1981; 80: 535–561.
Aronson Jr NN, Barrett AJ . The specificity of cathepsin B. Hydrolysis of glucagon at the C-terminus by a peptidyldipeptidase mechanism. Biochem J 1978; 171: 759–765.
Maciewicz RA, Etherington DJ, Kos J, Turk V . Collagenolytic cathepsins of rabbit spleen: a kinetic analysis of collagen degradation and inhibition by chicken cystatin. Coll Relat Res 1987; 7: 295–304.
Mason RW . Interaction of lysosomal cysteine proteinases with alpha 2-macroglobulin: conclusive evidence for the endopeptidase activities of cathepsins B and H. Arch Biochem Biophys 1989; 273: 367–374.
Gocheva V, Joyce JA . Cysteine cathepsins and the cutting edge of cancer invasion. Cell Cycle 2007; 6: 60–64.
Wu C, Orozco C, Boyer J, Leglise M, Goodale J, Batalov S et al. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol 2009; 10: R130.
Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci USA 2004; 101: 6062–6067.
Su AI, Welsh JB, Sapinoso LM, Kern SG, Dimitrov P, Lapp H et al. Molecular classification of human carcinomas by use of gene expression signatures. Cancer Res 2001; 61: 7388–7393.
Pesonen S, Kangasniemi L, Hemminki A . Oncolytic adenoviruses for the treatment of human cancer: focus on translational and clinical data. Mol Pharmacol 2011; 8: 12–28.
Yan W, Kitzes G, Dormishian F, Hawkins L, Sampson-Johannes A, Watanabe J et al. Developing novel oncolytic adenoviruses through bioselection. J Virol 2003; 77: 2640–2650.
Gros A, Martinez-Quintanilla J, Puig C, Guedan S, Mollevi DG, Alemany R et al. Bioselection of a gain of function mutation that enhances adenovirus 5 release and improves its antitumoral potency. Cancer Res 2008; 68: 8928–8937.
Kuhn I, Harden P, Bauzon M, Chartier C, Nye J, Thorne S et al. Directed evolution generates a novel oncolytic virus for the treatment of colon cancer. PLoS One 2008; 3: e2409.
Uil TG, Vellinga J, de Vrij J, van den Hengel SK, Rabelink MJ, Cramer SJ et al. Directed adenovirus evolution using engineered mutator viral polymerases. Nucleic Acids Res 2010; 39: e30.
Fallaux FJ, Kranenburg O, Cramer SJ, Houweling A, Van Ormondt H, Hoeben RC et al. Characterization of 911: a new helper cell line for the titration and propagation of early region 1-deleted adenoviral vectors. Hum Gene Ther 1996; 7: 215–222.
Vellinga J, Uil TG, de Vrij J, Rabelink MJ, Lindholm L, Hoeben RC . A system for efficient generation of adenovirus protein IX-producing helper cell lines. J Gene Med 2006; 8: 147–154.
de Vrij J, van den Hengel SK, Uil TG, Koppers-Lalic D, Dautzenberg IJ, Stassen OM et al. Enhanced transduction of CAR-negative cells by protein IX-gene deleted adenovirus 5 vectors. Virology 2010; 410: 192–200.
Murakami P, McCaman MT . Quantitation of adenovirus DNA and virus particles with the PicoGreen fluorescent dye. Anal Biochem 1999; 274: 283–288.
Caravokyri C, Leppard KN . Constitutive episomal expression of polypeptide IX (pIX) in a 293-based cell line complements the deficiency of pIX mutant adenovirus type 5. J Virol 1995; 69: 6627–6633.
DeNardo GL, DeNardo SJ, Peterson JJ, Miers LA, Lam KS, Hartmann-Siantar C et al. Preclinical evaluation of cathepsin-degradable peptide linkers for radioimmunoconjugates. Clin Cancer Res 2003; 9: 3865S–3872S.
Uil TG, de Vrij J, Vellinga J, Rabelink MJ, Cramer SJ, Chan OY et al. A lentiviral vector-based adenovirus fiber-pseudotyping approach for expedited functional assessment of candidate retargeted fibers. J Gene Med 2009; 11: 990–1004.
Douglas JT, Rogers BE, Rosenfeld ME, Michael SI, Feng M, Curiel DT . Targeted gene delivery by tropism-modified adenoviral vectors. Nat Biotechnol 1996; 14: 1574–1578.
Durupt F, Koppers-Lalic D, Balme B, Budel L, Terrier O, Lina B et al. The chicken chorioallantoic membrane tumor assay as model for qualitative testing of oncolytic adenoviruses. Cancer Gene Ther 2011; doi:10.1038/cgt.2011.68.
Acknowledgements
We thank Masato Yamamoto (Department of Surgery, University of Minnesota, MN, USA) for providing us with the plasmid pShuttle-ΔE3-ADP-EGFP-F2. In addition, Ivo Que (Department of Endocrinology, Leiden University Medical Center, Leiden, Netherlands) is gratefully acknowledged for precious technical assistance in the biofluorescence experiments. This work was supported by the European Union through the 6th Framework Program GIANT (Contract No. 512087).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
de Vrij, J., Dautzenberg, I., van den Hengel, S. et al. A cathepsin-cleavage site between the adenovirus capsid protein IX and a tumor-targeting ligand improves targeted transduction. Gene Ther 19, 899–906 (2012). https://doi.org/10.1038/gt.2011.162
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2011.162