Abstract
The accumulation of abnormal protein aggregates is a major characteristic of many neurodegenerative disorders, including Parkinson’s disease (PD). The intracytoplasmic deposition of α-synuclein aggregates and Lewy bodies, often found in PD and other α-synucleinopathies, is thought to be linked to inefficient cellular clearance mechanisms, such as the proteasome and autophagy/lysosome pathways. The accumulation of α-synuclein aggregates in neuronal cytoplasm causes numerous autonomous changes in neurons. However, it can also affect the neighboring cells through transcellular transmission of the aggregates. Indeed, a progressive spreading of Lewy pathology among brain regions has been hypothesized from autopsy studies. We tested whether inhibition of the autophagy/lysosome pathway in α-synuclein-expressing cells would increase the secretion of α-synuclein, subsequently affecting the α-synuclein deposition in and viability of neighboring cells. Our results demonstrated that autophagic inhibition, via both pharmacological and genetic methods, led to increased exocytosis of α-synuclein. In a mixed culture of α-synuclein-expressing donor cells with recipient cells, autophagic inhibition resulted in elevated transcellular α-synuclein transmission. This increase in protein transmission coincided with elevated apoptotic cell death in the recipient cells. These results suggest that the inefficient clearance of α-synuclein aggregates, which can be caused by reduced autophagic activity, leads to elevated α-synuclein exocytosis, thereby promoting α-synuclein deposition and cell death in neighboring neurons. This finding provides a potential link between autophagic dysfunction and the progressive spread of Lewy pathology.
Similar content being viewed by others
Introduction
α-Synuclein is an abundant neuronal protein that is linked to several major neurodegenerative diseases, including Parkinson’s disease (PD), dementia with Lewy bodies and multiple system atrophy; these diseases are collectively referred to as α-synucleinopathies.1 The amyloid fibril form of α-synuclein is thought to be the major component of Lewy bodies. Genetic studies have shown that missense mutations and a multiplication of the α-synuclein gene are linked to rare forms of inherited PD, and these mutations lower the age of disease onset in carriers of the mutation.2, 3, 4, 5, 6, 7 α–Synuclein consists of 140 amino acids and is a natively unfolded protein that spontaneously develops into amyloid-like fibrils in vitro in a nucleation-dependent manner.8 However, some studies have suggested that native α-synuclein is a tetrameric oligomer and that disrupting the tetrameric conformation may be critical for amyloid formation.9 Increased expression of α-synuclein is associated with its aggregation in neuronal models, animals and humans.10 Recent neuropathological studies have indicated that α-synuclein pathology progresses in highly specific and predictable patterns, first occurring in a few discrete regions of the lower brain stem and the olfactory bulbs and then spreading into the larger areas of the brain.11
The accumulation of α-synuclein aggregates in cells is likely to be a consequence of a disrupted dynamic equilibrium between the production and removal of this protein. Inefficient clearance of α-synuclein, which occurs through the autophagy/lysosome pathway, leads to the accumulation of toxic aggregates in cells and in animal models.12, 13 Autophagy is a cellular process that degrades long-lived proteins and dysfunctional or superfluous organelles, releasing the degradation products into the cytoplasm to be reused by essential biosynthetic pathways.14 Autophagy is induced by various stresses that limit nutrient and oxygen levels and decrease the energy supply. Three major types of autophagy processes have been found to occur: macroautophagy, chaperone-mediated autophagy (CMA) and microautophagy. In macroautophagy, a double-membraned phagophore engulfs a portion of the cytoplasm and matures into an autophagosome, which subsequently fuses with lysosomes to be degraded. CMA directly translocates the unfolded protein across a lysosomal membrane by interacting with specific receptor Lamp-2A. Microautophagy is the direct uptake of cytoplasm into lysosomes by an invagination of the lysosomal membrane.
Reports indicate that α-synuclein is degraded by both macroautophagy and CMA. The inhibition of CMA leads to the accumulation of high-molecular-weight and detergent-insoluble species of α-synuclein.15, 16 Treatment by macroautophagy inhibitors can also facilitate the accumulation of α-synuclein aggregates.17
Although α-synuclein is considered to be primarily a cytosolic protein, recent studies have shown that it can be released from neuronal cells into the culture medium through an unconventional secretory pathway.18 This release of α-synuclein increases under various stress conditions, raising the cellular levels of α-synuclein aggregates.19, 20 Extracellular α-synuclein may be a mediator of aggregate spreading in PD and other related diseases, as it can be taken up by neighboring neuronal and glial cells and cause neurotoxicity and neuroinflammation, respectively.21, 22, 23, 24, 25 This hypothesis may explain the underlying mechanism of the spread of Lewy pathology as well as the cause of the host-to-graft propagation of Lewy bodies in the long-term mesencephalic transplants of PD patients.26, 27
Here, we show that autophagic dysfunction, induced by pharmacological inhibitors or by the knockout of autophagy genes, results in the accumulation of α-synuclein aggregates in vesicle fractions and an increase in the exocytosis of α-synuclein. Under the same conditions, we also observed increased transcellular transfer of α-synuclein and elevated apoptotic cell death in the recipient cells.
Materials and methods
Materials
The primary antibodies used were as follows: α-synuclein monoclonal antibodies 62 and 274 from our laboratory,28 a Syn1 antibody (BD Biosciences, San Diego, CA, USA), ATG7 and cleaved caspase-3 antibodies (Cell Signaling Technology, Danvers, MA, USA) and a monoclonal β-actin antibody (Sigma, St Louis, MO, USA). Retinoic acid, 3-methyladenine (3-MA), rapamycin, 2-mercaptoethanol and a protease inhibitor cocktail were purchased from Sigma.
Cloning and production of the α-synuclein lentiviral vector
Human wild-type α-synuclein complementary DNA (cDNA) was amplified by PCR using the primer sets 5′-GATCCTAGAGCCACCATGGATGTATTCATGAAAGG-3′ and 5′-GATCGCGGCCGCGGCTCAGGTCCGTAGCCTTG-3′ and the α-synuclein/pcDNA3.1 vector as a template. The amplified product was digested with XbaI and NotI and inserted into the XbaI/NotI sites of the pcDH-EF1-MCS-T2A-copGFP vector (System Biosciences, Mountain View, CA, USA), and then sequenced for confirmation.
The cloned α-synuclein lentiviral vector (lenti/α-syn) was used to produce a recombinant α-synuclein lentivirus. Human embryonic kidney cells, line 293T, were grown in Dulbecco’s modified Eagle’s medium (Hyclone, Logan, UT, USA) with 10% fetal bovine serum without antibiotics, and 3 × 106 cells were plated on a 10-cm plate. The cells were transfected the next day with 2 μg of the lenti/α-syn vector and 10 μg of a pPACKH1-plasmid mix (System Biosciences) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). After 48 h of transfection, the medium was recovered and centrifuged at 1500 × g for 30 min to remove any cellular debris. The supernatant was further centrifuged at 50 000 × g for 2 h to harvest the virus. The pellet was resuspended in phosphate-buffered saline (PBS) and aliquoted for storage at −80 °C.
Cloning and production of the tfLC3 recombinant adenoviral vector
The ptfLC3 plasmid was a kind gift from Dr Tamotsu Yoshimori. It was cut with NheI, and the blunt end was generated with T4 DNA polymerase (NEB, Ipswich, MA, USA). The plasmid was then cut again with EcoRI. The tandem fluorescent-tagged LC3 (tfLC3) fragment was ligated into the SmaI/EcoRI site of a pDNR-CMV vector (BD Biosciences). Recombinant tfLC3 adenoviral DNA (adeno/tfLC3) was generated using the Adeno-X Expression System (BD Biosciences).
Cell culture and α-synuclein expression
The human neuroblastoma cell line SH-SY5Y was maintained and differentiated as described previously.12
Mouse embryonic fibroblast (MEF; a kind gift from Dr Komatsu) cell lines, both wild-type and ATG7 homozygous knockouts, were cultured at 37 °C in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, non-essential amino acids (Invitrogen), 0.1 μM 2-mercaptoethanol and penicillin/streptomycin (Invitrogen).
α-Synuclein expression
A recombinant adenoviral vector containing human α-synuclein cDNA (adeno/α-syn) was used to express α-synuclein in differentiated SH-SY5Y cells, as described previously.12 Differentiated SH-SY5Y cells were transduced with adeno/α-syn at a multiplicity of infection of 33 in a half volume of fresh growth medium. Following 90 min of incubation, the remaining half volume of fresh growth medium was added, and the cells were incubated overnight. The next day, the medium was replaced with fresh medium and incubated further before treatment.
To express α-synuclein in MEF cells, lenti/α-syn was added to the cells during seeding, and the medium was replaced with fresh growth medium the next day. On day 2 of the transduction, the medium was replaced with serum-free medium, and the conditioned medium was collected at the indicated times.
Co-culture experiments
For a mixed culture containing α-synuclein-expressing cells and wild-type cells, differentiated SH-SY5Y cells that had been transduced with recombinant adeno/α-syn (donor cells on day 1 of infection) were added to differentiated SH-SY5Y cells labeled with the Qtracker595 labeling kit (Invitrogen) (recipient cells) and cultured. After 2 days, the growth medium was replaced with serum-free medium, and 5 mM 3-MA was added. The culture was then incubated for 18 h.
Treatment of 3-MA
Differentiated SH-SY5Y cells infected with adeno/α-syn were treated with 1, 2.5, 5 and 10 mM of 3-MA on day 3 of the infection for 18 h or as indicated.
Inhibition of exocytosis
To inhibit general exocytosis, low-temperature incubation was utilized. Differentiated SH-SY5Y cells expressing α-synuclein in 35-mm dishes were treated with 0 or 10 mM 3-MA in Dulbecco’s modified Eagle’s medium without serum and then incubated for 16 h at 37 °C. After the cells were washed with Dulbecco’s modified Eagle’s medium, fresh medium containing 0 or 10 mM 3-MA was added, and the cells were incubated at 16 °C or 37 °C for 2 h.
Preparation of cell extracts and conditioned medium
Cell extracts were obtained as described previously.29 Cells were washed with PBS, and then the extraction buffer (1% Triton X-100 and the protease inhibitor cocktail in PBS) was added. After incubation on ice for 10 min, the cell extract was centrifuged at 16 000 × g for 10 min, and the supernatant and pellets were obtained for further analysis. The conditioned medium was centrifuged at 1000 × g for 10 min, and the transferred supernatant was spun again at 10 000 × g for 20 min. The recovered supernatant was stored with the protease inhibitor cocktail at −80 °C for further analysis.
Western blotting
Western blotting was performed as described previously.29 Chemiluminescence detection was performed using the FUJIFILM Luminescent Image Analyzer LAS-3000 and Multi Gauge (v3.0) software (FUJIFILM, Tokyo, Japan).
α-Synuclein ELISA
An α-synuclein enzyme-linked immunosorbent assay (ELISA) was performed as described previously.28 Ninety-six-well ELISA plates (Maxisorp; Nunc, Rochester, NY) were coated overnight at 4 °C with 1 μg ml−1 of capture antibody (62 antibody) in 50 mM carbonate buffer (pH 9.6). After the plates were washed in PBS with 0.05% Tween-20 (PBST), the blocking buffer was added and the plates were incubated for 1 h at room temperature (RT) with modest shaking. The plates were again washed in PBST, and the samples and standards were incubated at RT for 2.5 h with modest shaking. After a third washing with PBST, 1 μg ml−1 of biotinylated reporter antibody (274 antibody) in blocking buffer was added at RT for 1.5 h. The plates were again washed in PBST, and avidin-conjugated peroxidase (ExtrAvidin; Sigma) was added in a 1:1500 dilution in blocking buffer for 1 h at RT. After a final washing with PBST, 100 μl of the chemiluminescent peroxidase substrate (Sigma) solution mixture was added, and the plates were incubated at RT for 10 min in the dark before being read by a luminometer.
Total vesicle preparation
The procedure for vesicle preparation has been described previously.18 Buffer M (10 mM HEPES at pH 7.2, 10 mM KCl, 1 mM EGTA and 250 mM sucrose) and a protease inhibitor cocktail were used for harvesting cells. The cells were disrupted using a Dounce homogenizer and centrifuged at 1000 × g; then, the supernatant was mixed with OptiPrep density gradient medium (Sigma) to obtain a final concentration of 35%. The mixture was layered under 30 and 5% step-gradient layers. After centrifugation at 200 000 × g for 2 h, the total vesicles were collected at the interface between the 5 and 30% layers, and the cytosolic fraction was collected from the 35% bottom layer.
Cell viability assay
Cells were trypsinized and divided into two tubes. Detergent was added to one of the tubes to make the cells permeable. Propidium iodide was then added to both tubes to label the total cells (with added detergent) and the damaged cells (no detergent). Cells from both tubes were counted by an ADAM cell counter (NanoEnTek, Seoul, Korea). To determine the membrane integrity of the cells used in the experiments, the levels of released lactate dehydrogenase were measured in the culture supernatant, as described in the manufacturer’s instructions (Promega, Madison, WI, USA).
Digitonin permeabilization of cells
To observe the amount of membrane-bound LC3 in the tfLC3-overexpressing cells, the cells were permeabilized briefly with digitonin as described previously.30 The cells were rinsed in Hank’s balanced salt solution twice and incubated with 30 μM digitonin at RT for 5 min. The cells were then washed with Hank’s balanced salt solution and fixed in 4% paraformaldehyde for cell staining.
Immunofluorescence cell staining
The cell staining procedure has been described elsewhere.29 Cells grown on poly-L-lysine-coated coverslips were fixed in 4% paraformaldehyde in PBS. The fixed cells were permeabilized with 0.1% Triton X-100 before being incubated in blocking solution (5% bovine serum albumin and 3% goat serum in PBS). Primary antibodies were diluted in blocking solution and added to the cells, which were then incubated at RT for 30 min. After being washed in PBS, the cells were incubated with fluorescent dye Cy2-conjugated and rhodamine red X-conjugated secondary antibodies (Jackson Immunoresearch Laboratories, West Grove, PA, USA) and then washed again in PBS. The nuclei were stained with TOPRO-3 dye (Invitrogen), and the coverslips were mounted on slides in Antifade reagent (Invitrogen). The cells were observed under an Olympus FV1000 confocal laser scanning microscope (Center Valley, PA, USA).
Statistical analysis
All experiments were blind-coded and repeated three to four times. The values in the figures are expressed as the mean±s.e.m. Null hypotheses of no difference were rejected if P-values were <0.05. The graphs were drawn with Prism 5 software (Graphpad Software, La Jolla, CA, USA). To determine statistical significance, the values were compared by one-way analysis of variance with Tukey’s post test using InStat (version 3.05) software (Graphpad Software).
Results
Inhibiting autophagosome formation increases α-synuclein aggregation and secretion
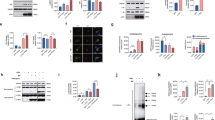
Differentiated SH-SY5Y neuroblastoma cells expressing human α-synuclein were treated with 3-MA, an inhibitor of autophagy-specific type-III phosphoinositide 3 kinases and Vps34 complexes. 3-MA inhibits the production of phosphatidylinositol-3-phosphate, which is important for autophagosome formation, thus inhibiting autophagy. We administered 3-MA to cells that were in a starving condition, which promotes starvation-induced autophagy, as 3-MA has been shown to promote autophagy under a nutrient-rich state.31 The 3-MA treatment increased the levels of sodium dodecyl sulfate-stable α-synuclein aggregates in both the triton-soluble and -insoluble fractions of the cells (Figure 1a). When the culture media were analyzed, we found that 3-MA increased the secretion of α-synuclein (Figure 1a). On the other hand, an autophagy-promoting condition induced by the mammalian Target of Rapamycin (mTOR) inhibitor rapamycin resulted in decreased secretion of α-synuclein (Figure 1b). The secretion of α-synuclein occurs progressively over time, and the extent of the secretion strongly correlated with the concentration of 3-MA given to the cells (Figure 2a). The increase in α-synuclein secretion was not due to membrane leakage; cell viability measurements based on membrane integrity showed no changes with 3-MA treatment (Figure 2b). The inhibitory effect of 3-MA on autophagy was confirmed by observing the autophagosome marker LC3 in the treated cells. Additionally, mRFP-GFP tfLC3 (Kimura et al. 32) was expressed in the SH-SY5Y cells expressing α-synuclein by transduction with the recombinant adenoviral vector (adeno/tfLC3). Autophagosome-bound tfLC3 has been shown to express both green fluorescent protein (GFP) and monomeric red fluorescent protein (mRFP) signals, but it loses the GFP signal after fusion with the lysosome.32 The cells were treated with 10 mM 3-MA for 18 h, and the cytosolic-free tfLC3 was removed by digitonin permeabilization to allow clearer observation of the membrane-bound tfLC3. The control cells displayed GFP and mRFP punctuates from the tfLC3, which often colocalized in the cells. However, the cells treated with 3-MA had significantly reduced GFP and mRFP signals, and the GFP signals were particularly weak (Figure 2c). This result could be explained by inhibition of autophagosome formation by 3-MA, which would cause a considerable decrease in the binding of tfLC3 to autophagosomes. The residual mRFP signals likely results from the tfLC3 that was delivered to lysosomes by the autophagosomes that survived the 3-MA inhibition.
Increased α-synuclein aggregation and secretion occur when autophagy is inhibited. Differentiated SH-SY5Y cells expressing α-synuclein were treated with dimethyl sulfoxide (DMSO) and either 3-methyladenine (3-MA; 1 or 10 mM) (a) or rapamycin (Rap) (b). The α-synuclein aggregation was compared between triton-soluble and triton-insoluble fractions as well as in the culture medium. Arrows indicate the monomeric size of α-synuclein, and brackets indicate the aggregated α-synuclein. β-Actin was used to demonstrate an equal loading of proteins in all blots (*P<0.05, ***P<0.001).
Dose-dependent increase of α-synuclein release in 3-methyladenine (3-MA)-treated cells. (a) Differentiated SH-SY5Y cells expressing α-synuclein were treated with increasing doses of 3-MA (0–10 mM), and the amount of α-synuclein released into the culture medium was measured by enzyme-linked immunosorbent assay (ELISA). (b) The graph shows the percentage of surviving cells after 3-MA treatment. (c) The inhibitory effect of 3-MA was confirmed by tandem fluorescent-tagged LC3 (tfLC3) expression. Cells expressing α-synuclein were treated with 10 mM 3-MA for 18 h and digitonin permeabilized before fixation. The graph on the right shows the reduced tfLC3 fluorescence after 3-MA treatment (scale bar: 20 nm; *P<0.05). GFP, green fluorescent protein; RFP, red fluorescent protein.
The role of autophagy in α-synuclein secretion was further investigated by genetically modifying the pathway. To this end, we used immortalized MEFs derived from wild-type and ATG7 knockout mice.33 The ATG7 protein acts as an E1-like enzyme and is required for the lipidation of LC3. It also conjugates ATG12 to ATG5/3, which is essential for autophagosome formation. A lack of ATG7 protein expression was confirmed in the ATG7 knockout MEFs by western blotting (Figure 2a). In these MEF cells, α-synuclein was expressed using lentiviral vectors, and an equal amount of expression was found in both wild-type and ATG7 knockout MEFs (Figure 2b). The secretion of α-synuclein was analyzed at different times using an ELISA for α-synuclein in the culture media. The secretion increased over time in both wild-type and ATG7 knockout MEFs. However, the levels of secreted α-synuclein increased more dramatically in ATG7 knockout MEFs than in wild-type MEFs, and there was an approximately threefold higher amount of secreted α-synuclein in the culture medium of ATG7 knockout MEFs at 24 h (Figure 3c). The increased α-synuclein release from ATG7 knockout MEFs was not due to increased cell death, as the cell viability of these cells did not differ from that of the wild-type MEFs after 24 h (Figure 3d). From these results of the chemical and genetic modulation of autophagy, as shown in Figures 1, 2, 3, we can conclude that a reduction in autophagic activity causes an increase in α-synuclein secretion.
α-Synuclein release in ATG7 knockout (KO) mouse embryonic fibroblast (MEF) cells. (a) Wild-type and ATG7 KO MEF cell extracts were immunoblotted against ATG7 to confirm its expression. (b) A recombinant α-synuclein lentivirus was used to express human α-synuclein in MEF cells. (c) The amounts of α-synuclein released in wild-type and ATG7 KO MEF cells were compared in a time-dependent manner (***P<0.001). (d) The percentage of surviving cells after 24 h. β-Actin was used to demonstrate an equal loading of proteins in all blots.
Involvement of exocytosis in α-synuclein secretion upon autophagy inhibition
Previous studies have suggested that α-synuclein is released from cells via exocytosis.18 This suggestion was based on observations that α-synuclein secretion was temperature sensitive and that a small amount of intracellular α-synuclein was present in the lumen of vesicles. To verify the involvement of exocytosis in the increased α-synuclein secretion of autophagy-compromised cells, we assessed the temperature sensitivity and vesicle localization of α-synuclein in the presence of 3-MA. An ELISA measurement showed that α-synuclein secretion was significantly decreased at 16 °C compared with the secretion at 37 °C in both the control and 3-MA-treated cultures of differentiated SH-SY5Y cells (Figure 4a). When this experiment was performed, no significant difference in cell viability was observed (Figure 4b). Next, we measured changes in the vesicular localization of α-synuclein. Upon treatment with 3-MA, the levels of α-synuclein in the vesicle fractions increased significantly (Figures 4c and d). These results suggest that the increased α-synuclein secretion occurring upon autophagic suppression is mediated by vesicle-mediated exocytosis.
Exocytosis-dependent secretion of α-synuclein in 3-methyladenine (3-MA)-treated cells. (a) Differentiated SH-SY5Y cells expressing α-synuclein were treated with dimethyl sulfoxide (DMSO) or 10 mM 3-MA and incubated at a normal (37 °C) or low (16 °C) temperature to inhibit exocytosis for 2 h. (a) The temperature-dependent release of α-synuclein to the medium (***P<0.001). (b) The cell survival percentage at low temperature. (c) The increase in α-synuclein aggregates in the vesicle fraction of 3-MA-treated cells is shown. The arrow represents the size of α-synuclein monomers, and the brackets show the aggregated forms of α-synuclein. (d) The graph shows the fold increase of α-synuclein in the vesicle fractions (*P<0.05).
Increased transcellular transmission of α-synuclein upon autophagy inhibition and subsequent toxicity
Our recent study demonstrated that secreted α-synuclein could be transferred to neighboring cells, accumulating to form various types of protein deposition, some of which resembled Lewy bodies.23 The cellular transfer of α-synuclein can be assessed in a mixed culture system of differentiated SH-SY5Y cells, in which donor cells overexpressing α-synuclein (stained green; no red dots) are co-cultured alongside recipient cells labeled with Qtracker595 (red dots; Figure 5a). When the mixed culture was treated with 3-MA, the puncta of the transferred α-synuclein in recipient cells were greater in size and number (Figure 5a), elevating the overall fluorescence intensity of α-synuclein in the recipient cells (Figure 5b). Although we have shown increased α-synuclein secretion due to 3-MA treatment in previous figures, we cannot rule out the possibility that the recipient cells were also affected by 3-MA in the mixed culture system. The diminished autophagic activity associated with common neurodegenerative disorders in humans, however, would affect not only the donor cells releasing the toxic protein aggregates but also the recipient cells that would have to clear the transferred aggregates. The increased transference of α-synuclein shown in Figure 5 is consistent with previous observations, indicating that autophagic defects promote the secretion of α-synuclein from donor cells. However, this observation does not rule out the possibility that the recipient cells have inhibited clearance of the transferred protein.
Autophagy inhibition increases the uptake and aggregation of α-synuclein in the recipient cells. (a) A mixed culture of differentiated SH-SY5Y cells expressing α-synuclein (donor cells) and nonexpressing cells labeled with Qtracker595 (recipient cells; red dots) are shown. Note the increased intensity and punctate sizes of α-synuclein staining in the 3-methyladenine (3-MA)-treated cells (yellow boxes). Green, α-synuclein; red, Qtracker595; blue, nuclei (scale bar: 20 μm). (b) The quantification of α-synuclein uptake in recipient cells (*P<0.05).
Neurotoxicity due to transmitted α-synuclein has been shown previously in cultured primary cortical neurons, which demonstrated nuclear fragmentation and caspase-3 activation.23 To determine whether increased α-synuclein secretion caused by autophagy inhibition and its subsequent uptake could affect the survival of the recipient cells, we compared the levels of cleaved caspase-3 in recipient cells with or without 3-MA treatment (Figure 6). SH-SY5Y cells expressing either α-synuclein or β-galactosidase (lacZ) were co-cultured with recipient cells labeled with Qtracker595 (red dots). The recipient cells cultured with lacZ-expressing donor cells showed little activation of caspase-3, and 3-MA treatment did not increase caspase-3 activation. This finding indicates that the 3-MA treatment itself is not toxic to cells under the conditions used in this experiment. The recipient cells cultured with α-synuclein-expressing donor cells, however, showed a significant increase in cleaved caspase-3 compared with the cells cultured with lacZ-expressing cells. The autophagy inhibition caused by 3-MA treatment further elevated caspase-3 activation compared with the nontreated cells, demonstrating that increased transmission of α-synuclein coincides with increased apoptotic cell death. These results demonstrate that autophagy inhibition causes increased α-synuclein secretion from a neuronal cell model, which leads to enhanced cell-to-cell α-synuclein transmission and coincidental cell death in the recipient cells.
Autophagy inhibition elevates cellular toxicity in the recipient cells. (a) A mixed culture of differentiated SH-SY5Y cells expressing α-synuclein (donor cells) and nonexpressing cells labeled with Qtracker595 (recipient cells; red dots) are shown. Note the increased cleaved caspase-3 staining (green) in the 3-methyladenine (3-MA)-treated cells. Green, cleaved caspase-3; red, Qtracker595; light blue, α-synuclein (scale bar: 20 μm). (b) The quantification of caspase activation in the recipient cells (*P<0.05).
Discussion
Basal, constitutive autophagy is essential for neuronal survival and differentiation.14 In many neurodegenerative diseases, autophagic vesicles often accumulate in neurons. It is not yet clear whether this accumulation is a sign of increased autophagic activity, reflecting a neuron’s effort to protect itself, or is a consequence of impaired autophagic flux that may result from dysfunction of specific autophagic machineries. However, recent reports have shown that diminished autophagic activity is associated with the accumulation of toxic protein aggregates in models of common neurodegenerative disorders, such as Alzheimer’s disease, Huntington’s disease and PD, suggesting that defective autophagy rather than corrective autophagy stimulation promotes neuronal death.34
Autophagy inhibition of both macroautophagy and CMA leads to the accumulation of high-molecular-weight and detergent-insoluble species of α-synuclein.15, 16, 17 In the present study, we hypothesized that autophagy inhibition also affects the transmission of α-synuclein from one cell to another, possibly causing toxic effects in neighboring cells. We used two cell models to investigate this problem: differentiated SH-SY5Y human neuroblastomas, a neuron-like cell line, treated with 3-MA, and a MEF cell line deficient in the autophagy gene ATG7. Autophagy inhibition, induced by either a chemical inhibitor or genetic modification, increased the accumulation of α-synuclein aggregates in cells and their subsequent release to the medium in a time- and dose-dependent manner. Our previous results have shown that stress conditions, which promote oxidative protein damage and inhibit the degradation of misfolded proteins, induce the vesicle translocation and release of α-synuclein.19, 20 Our current results are consistent with this previous study, showing that the diminished degradation of α-synuclein not only causes its accumulation in cells but also an increase in its release into the extracellular space.
The latest research on unconventional protein secretion has uncovered a novel secretory pathway termed ‘exophagy’, which involves the intermediate compartments in the autophagy pathway. In yeast, the secretion of the acyl coenzyme A-binding protein, Acb1, required both autophagy genes and genes for fusion with the plasma membrane.35, 36 A newly proposed mechanism for exophagy illustrates the fusion of an autophagosome with multivesicular bodies to form amphisomes, which then fuse with the plasma membrane to release their contents as either exosomes or nonmembrane-bound components. Recent studies on α-synuclein secretion have shown that α-synuclein can be released via exosomes.37, 38 A rise in calcium levels and in lysosomal dysfunction increased α-synuclein secretion in this manner. However, recovering α-synuclein in exosomes is very rare, in both our studies and others, and much of the extracellular α-synuclein is recovered as free protein (data not shown). Hasegawa et al. 39 showed that α-synuclein partially localizes with endosomal markers and the inhibition of VPS4, a protein that is required for the formation of intraluminal vesicles and exosomes, interfered with the lysosomal targeting of α-synuclein and increased its secretion, indicating that the exosomal pathway may not be solely responsible for α-synuclein release.
The release of α-synuclein occurs through temperature-dependent, brefeldin A-independent exocytosis, which is a characteristic of unconventional exocytosis.18 Our current results showed that inhibiting the early autophagy step of autophagosome formation increased α-synuclein secretion, indicating that α-synuclein release from cells does not necessarily require autophagosome formation. Therefore, there seems to be an unconventional exocytotic mechanism distinct from exophagy for α-synuclein secretion. However, this does not rule out the possibility that under normal conditions, the exophagy pathway plays a significant role in α-synuclein secretion.
Another recent interesting report described the inhibition of macroautophagy by α-synuclein.40 The study showed that α-synuclein compromised autophagy via Rab1a inhibition. This causes the mislocalization of Atg9, an important autophagy gene required for the formation of an autophagosome precursor, an omegasome. We also observed decreased LC3 staining in α-synuclein-expressing SH-SY5Y cells compared with lacZ-expressing cells (data not shown). Autophagic failure induced by α-synuclein would result in an accumulation of toxic proteins, including α-synuclein aggregates themselves, as well as mitochondrial dysfunction through mitophagy inhibition and other cellular dysfunctions. Therefore, the overproduction of α-synuclein might also lead to an increase in α-synuclein secretion through autophagy inhibition. This α-synuclein overproduction could be problematic, as elevated secretion results in increased transmission of α-synuclein to neighboring cells. Indeed, we showed that autophagic inhibition increased the cell-to-cell transmission of α-synuclein and that this transcellular transmission of α-synuclein coincided with greater apoptotic cell death in the recipient cells. Thus, we speculate from these studies that an increase in intracellular α-synuclein during cellular stresses leads to inhibition of autophagy, thereby causing an increase in the cell-to-cell transmission of α-synuclein and the associated death of neighboring cells.
In conclusion, we have demonstrated that autophagic inhibition not only caused the intracellular accumulation of α-synuclein but also increased α-synuclein secretion, promoting the cell-to-cell transmission of this protein and the death of its recipient cells. Further in-depth studies are needed to elucidate the secretory mechanism of α-synuclein under both normal and stress conditions, which may or may not occur through the same pathway.
References
Cookson MR . The biochemistry of Parkinson’s disease. Annu Rev Biochem 2005; 74: 29–52.
Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 1997; 276: 2045–2047.
Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease [letter]. Nat Genet 1998; 18: 106–108.
Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J et al. alpha-Synuclein locus triplication causes Parkinson’s disease. Science 2003; 302: 841.
Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S et al. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet 2004; 364: 1167–1169.
Ibanez P, Bonnet AM, Debarges B, Lohmann E, Tison F, Pollak P et al. Causal relation between alpha-synuclein gene duplication and familial Parkinson’s disease. Lancet 2004; 364: 1169–1171.
Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol 2004; 55: 164–173.
Uversky VN . Neuropathology, biochemistry, and biophysics of alpha-synuclein aggregation. J Neurochem 2007; 103: 17–37.
Bartels T, Choi JG, Selkoe DJ . alpha-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature 2011; 477: 107–110.
Kim C, Lee S-J . Controlling the mass action of alpha-synuclein in Parkinson’s disease. J Neurochem 2008; 107: 303–316.
Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K . Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 2004; 318: 121–134.
Lee H-J, Khoshaghideh F, Patel S, Lee S-J . Clearance of alpha-synuclein oligomeric intermediates via the lysosomal degradation pathway. J Neurosci 2004; 24: 1888–1896.
Ravikumar B, Sarkar S, Rubinsztein DC . Clearance of mutant aggregate-prone proteins by autophagy. Methods Mol Biol 2008; 445: 195–211.
Lynch-Day MA, Mao K, Wang K, Zhao M, Klionsky DJ . The role of autophagy in Parkinson’s disease. Cold Spring Harb Perspect Med 2012; 2: a009357.
Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D . Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science 2004; 305: 1292–1295.
Vogiatzi T, Xilouri M, Vekrellis K, Stefanis L . Wild type a-synuclein is degraded by chaperone mediated autophagy and macroautophagy in neuronal cells. J Biol Chem 2008; 283: 23542–23556.
Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC . Alpha-Synuclein is degraded by both autophagy and the proteasome. J Biol Chem 2003; 278: 25009–25013.
Lee H-J, Patel S, Lee S-J . Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci 2005; 25: 6016–6024.
Jang A, Lee H-J, Suk JE, Jung JW, Kim KP, Lee S-J . Non-classical exocytosis of alpha-synuclein is sensitive to folding states and promoted under stress conditions. J Neurochem 2010; 113: 1263–1274.
Lee HJ, Baek SM, Ho DH, Suk JE, Cho ED, Lee SJ . Dopamine promotes formation and secretion of non-fibrillar alpha-synuclein oligomers. Exp Mol Med 2011.
Kim YS, TH Joh . Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson's disease. Exp Mol Med 2006; 38: 333–347.
Lee H-J, Suk JE, Bae EJ, Lee S-J . Clearance and deposition of extracellular alpha-synuclein aggregates in microglia. Biochem Biophys Res Commun 2008; 372: 423–428.
Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci USA 2009; 106: 13010–13015.
Lee H-J, Suk JE, Patrick C, Bae EJ, Cho JH, Rho S et al. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem 2010; 285: 9262–9272.
Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A et al. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 2011; 72: 57–71.
Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW . Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med 2008; 14: 504–506.
Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med 2008; 14: 501–503.
Lee HJ, Bae EJ, Jang A, Ho DH, Cho ED, Suk JE et al. Enzyme-linked immunosorbent assays for alpha-synuclein with species and multimeric state specificities. J Neurosci Methods 2011; 199: 249–257.
Lee H-J, Shin SY, Choi C, Lee YH, Lee S-J . Formation and removal of alpha-synuclein aggregates in cells exposed to mitochondrial inhibitors. J Biol Chem 2002; 277: 5411–5417.
Lee H-J, Suk JE, Bae EJ, Lee JH, Paik SR, Lee S-J . Assembly-dependent endocytosis and clearance of extracellular alpha-synuclein. Int J Biochem Cell Biol 2008; 40: 1835–1849.
Wu YT, Tan HL, Shui G, Bauvy C, Huang Q, Wenk MR et al. Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J Biol Chem 2010; 285: 10850–10861.
Kimura S, Noda T, Yoshimori T . Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy 2007; 3: 452–460.
Sou YS, Waguri S, Iwata J, Ueno T, Fujimura T, Hara T et al. The Atg8 conjugation system is indispensable for proper development of autophagic isolation membranes in mice. Mol Biol Cell 2008; 19: 4762–4775.
Banerjee R, Beal MF, Thomas B . Autophagy in neurodegenerative disorders: pathogenic roles and therapeutic implications. Trends Neurosci 2010; 33: 541–549.
Duran JM, Anjard C, Stefan C, Loomis WF, Malhotra V . Unconventional secretion of Acb1 is mediated by autophagosomes. J Cell Biol 2010; 188: 527–536.
Manjithaya R, Anjard C, Loomis WF, Subramani S . Unconventional secretion of Pichia pastoris Acb1 is dependent on GRASP protein, peroxisomal functions, and autophagosome formation. J Cell Biol 2010; 188: 537–546.
Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH et al. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci 2010; 30: 6838–6851.
Alvarez-Erviti L, Seow Y, Schapira AH, Gardiner C, Sargent IL, Wood MJ et al. Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol Dis 2011; 42: 360–367.
Hasegawa T, Konno M, Baba T, Sugeno N, Kikuchi A, Kobayashi M et al. The AAA-ATPase VPS4 regulates extracellular secretion and lysosomal targeting of alpha-synuclein. PLoS One 2011; 6: e29460.
Winslow AR, Chen CW, Corrochano S, Acevedo-Arozena A, Gordon DE, Peden AA et al. alpha-Synuclein impairs macroautophagy: implications for Parkinson’s disease. J Cell Biol 2010; 190: 1023–1037.
Acknowledgements
This work was supported by grants from the National Research Foundation of Korea funded by the Korean Government (no. 2009-0073566), the National Research Foundation (NRF) funded by the Korean Government (MEST; no. 2010-0015188) and the Korea Health Technology R&D Project of the Ministry of Health and Welfare, Republic of Korea (A111228).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Lee, HJ., Cho, ED., Lee, K. et al. Autophagic failure promotes the exocytosis and intercellular transfer of α-synuclein. Exp Mol Med 45, e22 (2013). https://doi.org/10.1038/emm.2013.45
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/emm.2013.45
Keywords
This article is cited by
-
Single-cell transcriptomics reveal extracellular vesicles secretion with a cardiomyocyte proteostasis signature during pathological remodeling
Communications Biology (2023)
-
Role of α-synuclein in microglia: autophagy and phagocytosis balance neuroinflammation in Parkinson’s disease
Inflammation Research (2023)
-
Polarized α-synuclein trafficking and transcytosis across brain endothelial cells via Rab7-decorated carriers
Fluids and Barriers of the CNS (2022)
-
A breakdown in microglial metabolic reprogramming causes internalization dysfunction of α-synuclein in a mouse model of Parkinson’s disease
Journal of Neuroinflammation (2022)
-
TNF-α promotes α-synuclein propagation through stimulation of senescence-associated lysosomal exocytosis
Experimental & Molecular Medicine (2022)