Abstract
Fludarabine, a nucleoside analogue, is commonly used in combination with other agents for the treatment of chronic lymphocytic leukaemia (CLL). In previous studies, valproic acid (VPA), an inhibitor of histone deacetylases, combined with fludarabine to synergistically increase apoptotic cell death in CLL cells. In the present study, we found that the combination of fludarabine and VPA decreases the level of the anti-apoptotic proteins Mcl-1 and XIAP in primary CLL cells. Treatment with fludarabine alone, or in combination with VPA, led to the loss of lysosome integrity, and chemical inhibition of the lysosomal protease cathepsin B, using CA074-Me, was sufficient to reduce apoptosis. VPA treatment increased cathepsin B levels and activities in primary CLL cells, thereby priming CLL cells for lysosome-mediated cell death. Six previously treated patients with relapsed CLL were treated with VPA, followed by VPA/fludarabine combination. The combined therapy resulted in reduced lymphocyte count in five out of six and reduced lymph node sizes in four out of six patients. In vivo VPA treatment increased histone-3 acetylation and cathepsin B expression levels. Thus, the synergistic apoptotic response with VPA and fludarabine in CLL is mediated by cathepsin B activation leading to a decrease in the anti-apoptotic proteins.
Similar content being viewed by others
Introduction
Fludarabine-containing regimens are typically used in the frontline treatment of chronic lymphocytic leukaemia (CLL), but relapse is common and response rate with re-treatment is generally inferior to response as first-line treatment.1, 2, 3, 4 There is thus a need to develop new and non-cross-resistant drug combinations for relapsing patients. Inhibition of histone deacetylases (HDACs) using pharmacological agents, such as depsipeptide, suberoylanilide hydroxamic acid and valproic acid (VPA) is a novel strategy to treat such patients. In vitro results with HDAC inhibitors (HDIs) using depsipeptide, LBH589 and MS-275 were promising, implicating a number of different mechanisms associated with the inhibition of HDACs in CLL cells.5, 6, 7, 8 However, in the clinic, HDI monotherapy trials in CLL have been disappointing. A phase I trial with depsipeptide observed no responses, despite obvious increases in the level of acetylated histones and p21 in vivo.9 A phase II trial using MGCD0103 in previously treated CLL patients also produced no responses, despite some patients receiving concomitant rituximab.10 Although both depsipeptide and MGCD0103 were tolerable, haematological grade 4 toxicity was observed with both drugs.9, 10
As HDIs as monotherapy are not effective in CLL, we rationalized that VPA might be more useful if combined with chemotherapy. VPA is a first-generation antiepileptic that has class I HDAC inhibition activity with half-maximal inhibitory concentration values for HDAC1–3 of⩽1 mM.11, 12, 13 Treatment of CLL cells in vitro with VPA has been shown to induce apoptosis, as judged by caspase activation and Annexin V staining, whereas VPA inhibited proliferation of CLL cells induced by oligonucleotide and interleukin-2 co-stimulation.14, 15, 16 Single-agent VPA was sufficient to induce alterations in the gene expression level of a large number of genes and to change the Bcl-2/Bax ratio at the protein level.16, 17 Further, VPA enhanced the effects of various chemotherapeutic agents on CLL cells, including fludarabine, bortezomib, flavopiridol, thalidomide and lenalidomide.14, 16 Oral formulation, with high bioavailability, makes VPA easy to administer to patients, and doses of 20–25 mg/kg per day are commonly used in adolescents and adults.18, 19 As an antiepileptic, VPA has a long history in the clinic,19 and we reasoned that side effects would be predictable and manageable.
Lysosomes are involved in the cell death produced by a number of different antitumour drugs, including doxorubicin, camptothecin, cisplatin and etoposide.20, 21, 22 The mechanism of lysosome involvement in mediating cell death generally involves its disruption, a phenomenon known as lysosome membrane permeabilization (LMP).23, 24 LMP-associated cell death can be necrotic or apoptotic, and the mechanism of death is thought to be dependent on the degree of LMP, where partial LMP induces apoptosis.23, 24 Once disrupted, various lysosomal enzymes are spilled into the cytoplasm, some of which maintain their activity at physiological pH to mediate cell death. Among them, cathepsins B, D and L have been implicated in LMP-associated cell death.25 Cathepsin B is one of the most stable proteases at physiological pH and has been shown to mediate LMP-associated cell death in response to doxorubicin, bortezomib, tumour necrosis factor and during mammary involution in mice.26, 27, 28, 29 Released cathepsin B is active in the cytosol, where it can cleave many caspase targets and anti-apoptotic proteins, including Mcl-1 and XIAP.30, 31, 32 However, the role of lysosome-mediated cell death for the activity of antitumour agents in primary CLL cells is largely unstudied. In this study, we describe the role of cathepsin B in mediating VPA- and fludarabine-induced apoptosis in primary CLL cells.
Materials and methods
Cell culture and treatment conditions
The diagnosis of CLL was made by peripheral blood morphology and the presence of monoclonal B cells in the peripheral blood with typical immunophenotype (CD19+, CD5+ and CD23+). Peripheral blood samples were obtained from CLL patients following informed consent, in agreement with the Research Ethics Board at the University of Manitoba. Peripheral blood mononuclear cells were isolated from the buffy coat using a ficoll-paque density gradient as previously described.33 Freshly isolated CLL cells were cultured in Roswell Park Memorial Institute-1640 culture medium supplemented with 100 U of penicillin, 100 mg of streptomycin and 0.5% bovine serum albumin. Bovine serum albumin supplementation was chosen over fetal bovine serum supplementation for culturing of primary CLL cells, as bovine serum albumin supplementation was associated with lower levels of spontaneous apoptosis (Supplementary Figure S1). Three human B-cell leukaemia/lymphoma cell lines, BJAB, I-83 and NALM-6, were cultured in Roswell Park Memorial Institute-1640 culture medium supplemented with 100 U of penicillin, 100 mg of streptomycin and 10% fetal bovine serum.
Reagents used
Chloroquine, fludarabine, NH4Cl, VPA, propidium iodide and trichostatin A were purchased from Sigma-Aldrich (St Louis, MO, USA). Suberoylanilide hydroxamic acid was a gift from Dr Jim Davie (University of Manitoba). Antibodies against Mcl-1, XIAP and caspases-3, -8 and -9 were from Cell Signaling (Danvers, MA, USA). Antibodies against caspase-2 and cathepsin B were from Abcam (Toronto, ON, Canada). Antibodies against glyceraldehyde 3-phosphate dehydrogenase and tubulin were from Sigma (St Louis, MO, USA). Purified cathepsin B was purchased from Abcam. Chemical inhibitors of cathepsins, CA074-Me (Enzo Life Sciences, Farmingdale, NY, USA) and pepstatin A (Sigma), were also obtained commercially.
Cell death assays
Nuclear fragmentation was assessed by propidium iodide staining in hypotonic conditions and was performed with minor modifications using the direct DNA staining method.34 Briefly, cell pellets were resuspended in the propidium iodide staining buffer, supplemented with RNase (100 μg/ml). Cells were stained for 30 min at room temperature in the dark, after which samples were kept on ice and analysed on the flow cytometer within an hour.
Whole-cell lysates and immunoblotting
Whole-cell lysates were prepared from cell pellets, which were subjected to a single cycle of freeze thaw at −80 °C, and then resuspended in the radioimmunoprecipitation assay buffer supplemented with the Complete Mini protease inhibitor cocktail (Roche, Laval, QC, Canada), phosphatase inhibitor cocktails (Sigma-Aldrich) and sodium orthovanadate (1 mM, Sigma-Aldrich). Protein samples were quantified using either the Bradford (Bio-Rad, Mississauga, ON, Canada) or the bicinchoninic acid (Pierce, Rockford, IL, USA) protein quantification assay.
SDS-polyacrylamide gel electrophoresis was performed using standard protocols. Resultant blots were transferred onto nitrocellulose membranes and blocked using 5% milk or 5% bovine serum albumin in tris-buffered saline-Tween-20 (0.1% Tween-20). Blocked membranes were incubated overnight in the primary antibody mix, followed by incubation in the secondary antibody mix (horse radish peroxidase-conjugated anti-mouse/rabbit antibody (Bio-Rad). Enhanced chemiluminescence was performed using commercial reagents (GE Healthcare (Baie d'Urfe, QC, Canada) or Pierce).
Lysosome visualization
Lysosomes were stained using LysoTracker Red DND-99 (Life Technologies, Burlington, ON, Canada). Cells were stained with LysoTracker at 62.5 nM for 30 min at 37 °C and washed in fresh media before visualization or flow cytometry.
In vitro cathepsin B experiments
Whole-cell lysates were prepared in a lysis buffer containing NP40 supplemented with a protease inhibitor cocktail (Roche). Cell lysate protein was combined with ⩾0.2 U of cathepsin B, which was activated for 15 min at room temperature in the presence of dithiothreitol and phosphate-buffered saline (pH 7.4). The reaction mixtures were incubated at 37 °C, and the reaction was terminated by boiling the samples after the addition of SDS-polyacrylamide gel electrophoresis loading dye and dithiothreitol.
Benzyloxycarbonyl-Arg-Arg-7-amido-4-methylcoumarin (zRR-AMC) is a fluorogenic substrate of cathepsin B and was purchased from Enzo Life Sciences. Fresh lysates of treated cells were prepared using the NP40 buffer described above. Lysates were combined with phosphate-buffered saline containing zRR-AMC (100 μM) and incubated for 1 h at 37 °C. After an hour of incubation at 37 °C, zRR-AMC cleavage was measured using SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, CA, USA). To assess the level of background fluorescence, a control without lysate was used.
Phase II clinical trial
CLL patients aged 18 years or older who required therapy, had an ECOG (Eastern Cooperative Oncology Group) performance score of ⩽2 and had been previously treated with a nucleoside analogue were recruited into the trial. Patients were treated with oral VPA at a starting dose of 15 mg/kg per day orally in divided doses, with the goal of reaching a serum level of >1 mM through weekly therapeutic drug monitoring and dose escalation. For those with stable or progressive disease following 28 days of therapy, oral fludarabine at a dose of 40 mg/m2 per day on days 1–3 of each 28-day cycle was administered in conjunction with VPA. Treatment was continued to a maximum of six cycles. Therapy was discontinued if there was evidence of progressive disease after two cycles of fludarabine and VPA or if the patient experienced unacceptable toxicity attributable to the study drugs, for example, three or more non-haematological toxicity or prolonged grade 4 haematological toxicity NCI (National Cancer Institute criteria).
The primary outcome of interest was best clinical response as defined by the NCIWG (National Cancer Institute Working Group) criteria for CLL 6 months after commencing therapy.35 Secondary outcomes of interest were the effect of treatment on histone acetylation status and toxicity, both haematological and non-haematological. This trial conformed to good clinical practice guidelines and is registered at NCT00524667.
Statistical analyses
Analysis of variance was used to compare multiple columns, and t-tests were used for two-column comparisons (the Mann–Whitney). Analysis of variance was followed by post-test column comparison (Tukey’s test).
Results
A synergistic apoptotic response is observed when HDIs and fludarabine are combined
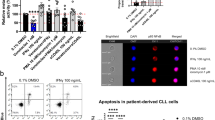
VPA has been shown to interact with fludarabine in a synergistic manner against primary CLL cells,14, 16 and this was confirmed when we examined primary CLL cells either co-treated or pretreated for 24 h with VPA and then treated with fludarabine (Supplementary Figure S1B), and similar results were obtained using either Annexin V staining or the sub-G1 method (Supplementary Figure S1D). There were no statistically significant differences in response to either fludarabine or the VPA/fludarabine combination in IgVH-mutated or unmutated CLL samples (Supplementary Figure S1C). We next examined three B-cell neoplasm-derived cell lines, BJAB, I-83 and NALM-6, which were chosen to represent the spectrum of different B-cell neoplasms, representing immature (NALM-6) to mature (CLL-like I-83) malignancies. Using sublethal dose of VPA (1 mM), the addition of VPA increased fludarabine cytotoxicity in all three cell lines (Figure 1a). This increased apoptosis was not observed between fludarabine and valpromide, the amide analogue of VPA that lacks the HDAC inhibition activity,36 suggesting that HDAC inhibition is important for synergy (Figure 1a). Fludarabine cytotoxicity was also increased by the addition of three different HDIs at sublethal doses, namely sodium butyrate, trichostatin A and suberoylanilide hydroxamic acid (Figure 1b). When the status of histone acetylation of histone-3 and histone-4 were examined, the VPA and fludarabine combination induced higher levels of histone acetylation than either agent alone (Supplementary Figure S1E). This was associated with an increase in the levels of histone-3 and histone-4 by fludarabine treatment, albeit to a greater extent in BJAB and NALM-6 cells compared with I-83 cells, as well as downregulation of HDAC1 by the VPA/fludarabine combination (Supplementary Figure S1E). The combination also induced activation of initiator caspases-2, -8 and -9 and the executioner caspase-3 in cell lines and primary CLL cells (Figure 1c), suggesting that both the intrinsic and extrinsic apoptotic pathways are activated.
Cytotoxicity of fludarabine is enhanced by HDIs. (a) Three human leukaemic cell lines, BJAB, NALM-6 and I-83, were treated for 1 day using combinations of fludarabine (5 μM) with valproic acid (VPA, 1 mM) or valpromide (VPA analogue that does not target HDACs, 1 mM). DNA fragmentation was quantified by flow cytometry, examining for hypodiploid DNA content. (b) Fludarabine (5 μM) was combined with three other HDIs, sodium butyrate (1 mM), trichostatin A (5 nM) or suberoylanilide hydroxamic acid (5 μM) in BJAB or NALM-6 cells for 1-day treatment. (c) Immunoblots using whole-cell lysates to examine caspase activation. Cell lines were treated for 1 day and primary CLL cells were treated for 2 days using combinations of fludarabine (5 μM for cell lines, 10 μM for primary CLL cells) and VPA (1 mM). Bar=mean, error bar=one s.e.m. Primary CLL data shown are representative data, and all experiments were repeated at least three independent times. *** represents significant differences in vehicles versus VPA treated cells in the presence of fludarabine.
The combination of VPA and fludarabine induces Mcl-1 and XIAP degradation in a lysosome-dependent manner
In CLL cells, various HDIs have previously been shown to downregulate the anti-apoptotic proteins, Bcl-2, Mcl-1 and XIAP.16, 17, 37 In the present study, we found that the levels of XIAP and Mcl-1 were reduced in both primary CLL and cell lines in response to fludarabine, and this reduction was further enhanced when fludarabine was combined with VPA (Figure 2a). In contrast, the Bcl-2 levels remained unchanged in primary CLL cells, whereas Bcl-2 was not expressed at detectable levels in BJAB or NALM-6 cells. In BJAB cells, the reduction in Mcl-1 and XIAP occurred with 16 h of treatment, and the same reduction was observed with 24-h treatment in primary CLL cells, and these observations were evident before significant apoptosis had occurred (Supplementary Figures S2A and B). This suggests that the downregulation of the proteins was contributing to the apoptosis. Interestingly, when the mRNA levels for these proteins were examined, there was no reduction in transcription, and the XIAP mRNA levels increased after treatment (Supplementary Figure S2C and D), suggesting that Mcl-1 and XIAP downregulation occurs post-transcriptionally.
The role of different protein degradation pathways in Mcl-1 and XIAP degradation. (a) Cell lines were treated for 1 day and primary CLL cells were treated for 2 days using combinations of fludarabine (5 μM for cell lines, 10 μM for primary CLL cells) and VPA (1 mM). Whole-cell lysates were examined for the levels of Bcl-2, Mcl-1 and XIAP in response to the indicated treatment combinations. Primary CLL data shown are representative data. (b) BJAB cells were pretreated with vehicles, chloroquine (an inhibitor of the lysosomal protein degradation), zVAD.fmk (a pan-caspase inhibitor) or bortezomib (a 26S proteasome inhibitor) for 1 h, and then treated with the indicated combinations of fludarabine and VPA for 16 h. Whole-cell lysates were examined by immunoblotting. The data shown for different treatments are obtained from a single representative experiment from the same membrane. All experiments were repeated three independent times.
Protein degradation can occur via a number of different pathways, including autophagy, lysosomal degradation and the ubiquitin–proteasome pathway.38, 39 Under apoptosis, a large number of proteins also become targeted by caspases.40 In order to investigate which pathways were involved in the degradation of Mcl-1 and XIAP, BJAB cells were pretreated with chloroquine (inhibitor of autophagy and lysosomal pathways), zVAD-fmk (pan-caspase inhibitor) or bortezomib (26S proteasome inhibitor). We determined whether these inhibitors could block protein degradation and caspase activation. Pretreatment with either chloroquine or zVAD-fmk reduced activation of caspases-2 and -8 and partially stabilized Mcl-1 and XIAP in response to fludarabine, in the presence or absence of VPA (Figure 2b). In contrast, bortezomib increased caspase activation in cells treated with either VPA or fludarabine and did not stabilize XIAP in response to the VPA/fludarabine combination (Figure 2b). Bortezomib did, however, increase the levels of Mcl-1, as a result of blockage of the ubiquitin–proteasome pathways (Figure 2b). Taken together, both lysosome and caspases appear to be involved in the degradation of XIAP and Mcl-1 by fludarabine, with or without VPA, and inhibition of the lysosomal protein degradation pathway suppressed caspase activation.
We next investigated the effects of lysosome alkalinization on the level of cell death. Pretreatment with chloroquine or NH4Cl reduced cell death in response to the combination of VPA and fludarabine in both BJAB and NALM-6 cells (Figure 3a). This protection by chloroquine or NH4Cl is not associated with inhibition of autophagy, as fludarabine, in the presence or absence of VPA, did not induce the formation of LC3-II (an indicator of autophagy), as determined by immunoblotting and was not associated with increase in the number of acidic vacuoles assessed by acridine orange staining (Supplementary Figures S3, S4A and B). Rather, the addition of chloroquine resulted in the increase in steady-state levels of LC3-II because of blockage of its degradation by lysosomes (Supplementary Figure S3). In primary CLL cells, pretreatment with chloroquine or NH4Cl reduced VPA/fludarabine- and fludarabine-induced apoptosis, although the magnitude of suppression was much lower, because of the toxicity associated with chloroquine or NH4Cl pretreatment (Figure 3b). In contrast to chloroquine or NH4Cl, treatment of BJAB or NALM-6 cells with MG-132 or bortezomib increased VPA and fludarabine cytotoxicity (Figure 3c). These observations suggest the lysosomes are having a crucial, autophagy-independent role in VPA/fludarabine-induced cell death.
Inhibition of the endolysosomal pathway protects cells from VPA-fludarabine cytotoxicity. (a, b) BJAB, NALM-6 or primary CLL cells were pretreated for 1 h with two different inhibitors of the lysosomal protein degradation pathway, chloroquine (100 μM) or NH4Cl (50 mM), and then treated for 1 (cell lines) or 2 (primary CLL cells) days using the indicated combinations of fludarabine (5 μM for cell lines, 10 μM for primary CLL cells) and VPA (1 mM). Data are gathered from 11 CLL patient samples. (c) BJAB or NALM-6 cells were pretreated with two inhibitors of the 26S proteasomal protein degradation pathway, MG-132 (1 μM) or bortezomib (1 nM), and then treated for 1 day using the indicated combinations of fludarabine (5 μM) and VPA (1 mM). Bar=mean, error bar=one s.e.m. All experiments in cell lines were repeated at least three independent times. * represents significant differences between vehicle and chloroquine treated cells in primary CLL cells. ** represents significant differences between vehicle and chloroquine or NH4CL in cell lines. *** represents significant differences between vehicle and NH4CL treatments in primary CLL cells.
Apoptosis induced by the combination of VPA and fludarabine is cathepsin B-dependent
The lysosome has been implicated in cell death pathways that involve its disruption, known as LMP, leading to the activation of caspases-2 and -8.23 As the VPA/fludarabine combination activated caspase-2 and lysosome alkalinization protected the cells from the combination, we next determined whether LMP is involved in cell death induced by VPA and fludarabine. In cell lines, staining of acidic compartments using acridine orange showed a loss of acidic compartments in response to fludarabine and VPA (Supplementary Figure S4A). When quantified, the percent of cells with loss of acidic compartments (that is, no detectable acridine orange staining) corresponded with the level of apoptosis (Supplementary Figure S4B). We next used LysoTracker, a commercial dye for acidic compartments, to examine primary CLL cells. LysoTracker staining showed a similar loss of acidic compartments in primary CLL cells following treatment, and the loss was visibly obvious by microscopy (Supplementary Figure S4C) and by flow cytometry (Figure 4a). When signals were quantified, LysoTracker signals were significantly reduced in either fludarabine- or VPA/fludarabine-treated cells (Supplementary Figure S4D).
The role of cathepsin B in VPA-fludarabine cytotoxicity. (a) Flow cytometric analysis of a representative CLL patient sample using LysoTracker staining of the lysosomes. Primary CLL cells were treated for 2 days using the indicated combinations of fludarabine (10 μM) and VPA (1 mM). Loss of lysosomal integrity is indicated by decrease in LysoTracker staining intensity. (b) BJAB or NALM-6 cells were pretreated for 1 h using CA074-Me, a permeable cathepsin B inhibitor or pepstatin A, a cathepsin D inhibitor, and then treated for 1 day using the indicated combinations of fludarabine (5 μM) and VPA (1 mM). (c) Whole-cell lysates from b examined via immunoblotting. (d) BJAB or NALM-6 cell lysates (10 μg each) was combined with activated, purified cathepsin B and incubated for the indicated durations. Reactions were stopped by adding SDS-polyacrylamide gel electrophoresis loading dye supplemented with dithiothreitol (50 mM) and boiling the samples for 5 min before analysis via SDS-polyacrylamide gel electrophoresis and immunoblotting. Bar=mean, error bar=one s.e.m. Primary CLL data shown are representative data, and all experiments in cell lines were repeated at least three independent times. ** represents significant differences between vehicle and pepstatin A treatments in cell lines.
Disruption of lysosomes, however, is a phenomenon that is observed in many different modes of cell death where lysosomes may or may not be having a driving role in the induction of cell death.41 When the disruption of lysosomes do actually drive cell death, such cell death scenarios involve the release of the lysosomal proteases into the cytosol and, among the different proteases, cathepsins B and D have been implicated in such cell deaths.23 When BJAB or NALM-6 cells were pretreated with CA074-ME, an inhibitor of cathepsin B, there was a robust protection of cells from fludarabine- or VPA/fludarabine-induced apoptosis, whereas much higher concentrations of pepstatin A, an inhibitor of cathepsin D, failed to show any protection (Figure 4b). Pretreatment of NALM-6 cells with CA-074ME partially stabilized Mcl-1 and XIAP and also reduced the activation of caspases, including caspase-2 (Figure 4c). Unlike cell lines, treatment of primary CLL cells with CA074-Me (20 μM) was toxic, but nonetheless resulted in mild suppression of the cytotoxicity seen with the VPA and fludarabine combination, where the difference approached statistical significance (Supplementary Figure S4E).
Although CA074-Me pretreatment partially stabilized Mcl-1 and XIAP, the pretreatment also inhibited the activation of caspases, which may be cleaving Mcl-1 and XIAP on their own. We thus determined whether cathepsin B could directly target Mcl-1 and XIAP, as cathepsin B has been shown to target purified Bcl-2 proteins, including Mcl-1 and XIAP in a cell-free system using purified proteins.32 We thus simulated LMP by combining whole-cell lysates from BJAB or NALM-6 cells with purified, activated cathepsin B to determine whether cathepsin B could directly degrade Mcl-1 and XIAP in vitro. When BJAB or NALM-6 lysates were mixed with purified cathepsin B, there were reductions in Mcl-1 and XIAP levels (Figure 4d), thus demonstrating that cathepsin B can target Mcl-1 and XIAP. Cathepsin B addition also resulted in the appearance of faster migrating bands with caspase-2 immunoblotting and a reduction in the pro-caspase-2 levels (Figure 4d). Taken together, treatment of leukaemic cells with fludarabine and VPA induces LMP, and the resulting apoptosis is dependent on the lysosomal protease cathepsin B, which can directly target Mcl-1, XIAP and caspase-2.
VPA upregulates cathepsin B levels and activity
A microarray study of VPA-treated CLL cells had reported cathepsin B to be upregulated.16 Treatment of BJAB cells with VPA resulted in increased levels of cathepsin B, which was not observed with valpromide (Figure 5a). We next asked whether this increase in cathepsin B level was also associated with increase in the cathepsin B activity. Fresh lysates from BJAB or NALM-6 cells that had been treated with either vehicle or 1 mM VPA was mixed with zRR-AMC, a fluorogenic substrate for cathepsin B whose cleavage product (7-amino-4-methylcoumarin) is fluorescent. Cleavage of zRR-AMC was detectable when combined with either vehicle-treated BJAB or NALM-6 lysates, and this activity was increased in lysates obtained from cells that had been treated for 24 h with 1 mM VPA (Figure 5b). Co-treatment of cells with CA074-Me (20 μM) reduced the fluorescence to the background level, demonstrating that zRR-AMC cleavage is cathepsin B dependent. In primary CLL cells, similar increase in cathepsin B levels in response to VPA was observable (Figure 5c), and VPA treatment was sufficient to increase zRR-AMC cleavage with lysates from primary CLL cells (Figure 5d). Thus, taken together, VPA increases cathepsin B protein levels, and this upregulation is associated with increased cathepsin B activity.
The effect of VPA on cathepsin B level and activity. (a) BJAB cells were treated using either VPA at indicated doses or valpromide (1 mM, VPA analogue that does not inhibit HDACs) for 1 day, and whole-cell lysates were examined. (b) BJAB or NALM-6 cells were treated for 1 day using the indicated combinations of VPA (1 mM) and CA074-Me (20 μM, a cathepsin B inhibitor), and then 40 μg of whole-cell lysates were combined with zRR-AMC, a fluorogenic substrate for cathepsin B in phosphate-buffered saline (pH 7.4). (c) Two primary CLL samples were treated using the indicated doses of VPA for 2 days, and whole-cell lysates were examined via immunoblotting. (d) Primary CLL cells were treated for 2 days using VPA (1 mM). Cells were collected and 15 μg of whole-cell lysates were combined with zRR-AMC as in b. The data are obtained from experiments with six different CLL samples. Bar=mean, error bar=one s.e.m. Primary CLL data shown are representative data, and all experiments were repeated at least three independent times. * represents significant differences between untreated and VPA treated primary CLL cells. ** represents significant differences between untreated and VPA treated cell lines in the presence of vehicle.
Phase I/II clinical trial data using a VPA and fludarabine combination
To determine whether VPA and fludarabine give increased clinical efficacy for CLL patients, we conducted a phase I/II clinical trial. Of six patients registered in the trial, three patients were fludarabine resistant and all had received a variety of chemotherapeutic regimens before trial entry (Table 1). Fludarabine resistance was clinically defined as having failed treatment or relapsed within 6 months of completing prior fludarabine-based therapy as per the international workshop on CLL guidelines.42 Four patients had Rai stage IV disease and two patients Rai stage III disease. Response with a 28-day cycle of VPA monotherapy was modest, with no response in half of the patients, and VPA was then combined with fludarabine for the remainder of the treatment course. Patients had VPA concentrations in the serum between 0.54–1.1 mM (Supplementary Table S1). Two patients had progressive disease following therapy, three patients had stable disease with >50% decrease in lymphocyte count following five cycles of therapy and one patient had a partial remission at the completion of six cycles of therapy (Figure 6a, Supplementary Table S2). Two patients completed all planned cycles of therapy. Two patients developed significant anaemia (NCI CTC grade 4) and one patient developed significant fatigue (NCIC CTC grade 3) during the fifth cycle of therapy necessitating early treatment discontinuation. Nevertheless, out of the six patients, five showed a decrease in lymphocyte count and lymph node sizes (Figures 6a and b).
Clinical and translational data. (a) Absolute lymphocyte count values of the six patients enrolled in the clinical trial. (b) Waterfall plot of best decrease in lymph node sizes. (c) Whole-cell lysates obtained from four patients while undergoing VPA monotherapy at indicated times. (d) Densitometry was performed on cathepsin B cells in CLL cells and normalized to glyceraldehyde 3-phosphate dehydrogenase.
To evaluate whether VPA is targeting histone acetylation and cathepsin B expression in vivo, peripheral blood samples were obtained during the first 30 days of the treatment course on VPA monotherapy and mononuclear cells were isolated for analysis by immunoblotting. Levels of both histone-3 acetyl initially increased and then fluctuated during the course of treatment (Figure 6c, Supplementary Figure S5). More importantly, cathepsin B expression was increased on average 2.3-fold in four patients’ CLL cells over a 30-day time course following VPA treatment (Figure 6d). The increase in cathepsin B levels were similar to those seen in VPA-treated primary CLL cells in vitro and corresponded to the subsequent decrease in lymphocyte count when fludarabine was added (Figure 6a).
Discussion
Although it has recently become apparent that the lysosome can contribute to cell death, this activity has been largely unstudied in CLL. The lysosome is the ultimate target for the TOSO:IgM complex in CLL cells, thereby contributing to the regulation of the BCR signalling pathway.43 In the context of rituximab, a type I anti-CD20 monoclonal antibody, the lysosome is the ultimate destination for FcγRIIB-promoted internalization of CD20:anti-CD20 complexes leading to reduced expression of CD20 on the cell surface, thereby contributing to rituximab resistance.44 On the other hand, GA101, a type II anti-CD20 monoclonal antibody, induces lysosome-dependent cell death, which was increased with CD40 ligation.45 Thus, lysosome-mediated cell death may be a novel mechanism to kill CLL cells.
In this study, we describe the involvement of the lysosome in fludarabine- and VPA/fludarabine-induced death of CLL cells. Although the GA101-induced cell death was described as being non-apoptotic,45 VPA with fludarabine treatment induced DNA fragmentation, phosphatidylserine externalization, nuclear condensation (data not shown) and the activation of multiple caspases, thus demonstrating that the cell death is apoptotic in nature. The difference between the treatments with GA101 versus VPA and fludarabine could be due to the extent of LMP. It is known that LMP can lead to both apoptosis and necrosis, depending on the extent of LMP; necrosis appears to be the dominant pathway of death in the setting of massive LMP, whereas partial LMP drives apoptotic cell death.23 GA101-induced LMP was rapid and obvious after hours of treatment,45 whereas fludarabine-induced LMP was much slower in comparison. By simulating LMP, we show that activated cathepsin B can target anti-apoptotic proteins, Mcl-1 and XIAP, as well as caspase-2. Considering the importance of the anti-apoptotic proteins in CLL cell survival, especially in the tumour microenvironment,46 targeting their degradation through cathepsin B could be a novel strategy to reduce drug resistance in the CLL microenvironment. In line with this hypothesis, we observed shrinkage of the lymph nodes in four of the six patients treated with the VPA/fludarabine combination, and we believe cathepsin B activation and release contributed to this clinical observation. The role of cathepsin B in the tumour microenvironment will be a focus of future investigations.
Some HDIs have also been described to cause LMP itself. Sulforaphane monotherapy has been observed to induce LMP,47 and the release of cathepsin B was found to contribute to the toxicity of doxorubicin when combined with HDIs.48 In this study, VPA upregulated cathepsin B levels in cell lines, primary CLL cells in vitro and in the leukaemic cells of CLL patients treated in the clinic, resulting in increased protease activity in cell lysates. As cathepsin B itself has been found to be important for the disruption of lysosomes,49 the increase in cathepsin B activity is likely to be sensitizing the lysosomes to LMP, as well as the subsequent cathepsin B-dependent protein degradation. In early studies that examined the biochemical activity of lysosomal enzymes in CLL, such as acid phosphatases and β-glucuronidases, peripheral blood CLL cells showed lower activity compared with normal lymphocytes,50 whereas another study showed CLL samples to have lower lysosomal acid phosphatase activity compared with other lymphomas.51 Although VPA had little activity when used alone in CLL patients, it increased cathepsin B levels in the cells of all four patients examined. In addition, there was a synergistic response between VPA and fludarabine in primary CLL cells in vitro, and as VPA produced a similar effect on the cathepsin B levels in CLL cells in treated patients, it is likely that VPA contributed to the clinical response. Thus, upregulation of cathepsin B levels would be a sensible and rational adjunct strategy for lysosome-targeted therapy.
References
Montserrat E, Moreno C, Esteve J, Urbano-Ispizua A, Giné E, Bosch F . How I treat refractory CLL. Blood 2006; 107: 1276–1283.
Keating MJ, O'Brien S, Kontoyiannis D, Plunkett W, Koller C, Beran M et al. Results of first salvage therapy for patients refractory to a fludarabine regimen in chronic lymphocytic leukemia. Leuk Lymphoma 2002; 43: 1755–1762.
Tsimberidou A-M, Keating MJ . Treatment of fludarabine-refractory chronic lymphocytic leukemia. Cancer 2009; 115: 2824–2836.
Badoux XC, Keating MJ, Wang X, O'Brien SM, Ferrajoli A, Faderl S et al. Fludarabine, cyclophosphamide, and rituximab chemoimmunotherapy is highly effective treatment for relapsed patients with CLL. Blood 2011; 117: 3016–3024.
Aron JL, Parthun MR, Marcucci G, Kitada S, Mone AP, Davis ME et al. Depsipeptide (FR901228) induces histone acetylation and inhibition of histone deacetylase in chronic lymphocytic leukemia cells concurrent with activation of caspase 8–mediated apoptosis and down-regulation of c-FLIP protein. Blood 2003; 102: 652–658.
Inoue S, MacFarlane M, Harper N, Wheat LMC, Dyer MJS, Cohen GM . Histone deacetylase inhibitors potentiate TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in lymphoid malignancies. Cell Death Differ 2004; 11: S193–S206.
Inoue S, Riley J, Gant TW, Dyer MJS, Cohen GM . Apoptosis induced by histone deacetylase inhibitors in leukemic cells is mediated by Bim and Noxa. Leukemia 2007; 21: 1773–1782.
Lucas DM, Davis ME, Parthun MR, Mone AP, Kitada S, Cunningham KD et al. The histone deacetylase inhibitor MS-275 induces caspase-dependent apoptosis in B-cell chronic lymphocytic leukemia cells. Leukemia 2004; 18: 1207–1214.
Byrd JC, Marcucci G, Parthun MR, Xiao JJ, Klisovic RB, Moran M et al. A phase 1 and pharmacodynamic study of depsipeptide (FK228) in chronic lymphocytic leukemia and acute myeloid leukemia. Blood 2005; 105: 959–967.
Blum KA, Advani A, Fernandez L, Van Der Jagt R, Brandwein J, Kambhampati S et al. Phase II study of the histone deacetylase inhibitor MGCD0103 in patients with previously treated chronic lymphocytic leukaemia. Br J Haematol 2009; 147: 507–514.
Gottlicher M, Minucci S, Zhu P, Kramer OH, Schimpf A, Giavara S et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J 2001; 20: 6969–6978.
Gurvich N, Tsygankova OM, Meinkoth JL, Klein PS . Histone deacetylase is a target of valproic acid-mediated cellular differentiation. Cancer Res 2004; 64: 1079–1086.
Bantscheff M, Hopf C, Savitski MM, Dittmann A, Grandi P, Michon A-M et al. Chemoproteomics profiling of HDAC inhibitors reveals selective targeting of HDAC complexes. Nat Biotech 2011; 29: 255–265.
Bouzar AB, Boxus M, Defoiche J, Berchem G, Macallan D, Pettengell R et al. Valproate synergizes with purine nucleoside analogues to induce apoptosis of B-chronic lymphocytic leukaemia cells. Br J Haematol 2009; 144: 41–52.
Lagneaux L, Gillet N, Stamatopoulos B, Delforge A, Dejeneffe M, Massy M et al. Valproic acid induces apoptosis in chronic lymphocytic leukemia cells through activation of the death receptor pathway and potentiates TRAIL response. Exp Hematol 2007; 35: 1527–1537.
Stamatopoulos B, Meuleman N, De Bruyn C, Mineur P, Martiat P, Bron D et al. Antileukemic activity of valproic acid in chronic lymphocytic leukemia B cells defined by microarray analysis. Leukemia 2009; 23: 2281–2289.
Bokelmann I, Mahlknecht U . Valproic acid sensitizes chronic lymphocytic leukemia cells to apoptosis and restores the balance between pro- and antiapoptotic proteins. Mol Med 2008; 14: 20–27.
Gerstner T, Bell N, König S . Oral valproic acid for epilepsy: long-term experience in therapy and side effects. Expert Opin Pharmacother 2008; 9: 285–292.
Perucca E . Pharmacological and therapeutic properties of valproate: a summary after 35 years of clinical experience. CNS Drugs 2002; 16: 695–714.
Paquet C, Sane AT, Beauchemin M, Bertrand R . Caspase- and mitochondrial dysfunction-dependent mechanisms of lysosomal leakage and cathepsin B activation in DNA damage-induced apoptosis. Leukemia 2005; 19: 784–791.
Emert-Sedlak L, Shangary S, Rabinovitz A, Miranda MB, Delach SM, Johnson DE . Involvement of cathepsin D in chemotherapy-induced cytochrome c release, caspase activation, and cell death. Mol Cancer Ther 2005; 4: 733–742.
Cho S, Park J, Hwang E . Kinetics of the cell biological changes occurring in the progression of DNA damage-induced senescence. Mol Cells 2011; 31: 539–546.
Boya P, Kroemer G . Lysosomal membrane permeabilization in cell death. Oncogene 2008; 27: 6434–6451.
Repnik U, Stoka V, Turk V, Turk B . Lysosomes and lysosomal cathepsins in cell death. Biochim Biophys Acta 2012; 1824: 22–33.
Turk V, Turk B, Turk D . Lysosomal cysteine proteases: facts and opportunities. EMBO J 2001; 20: 4629–4633.
Kreuzaler PA, Staniszewska AD, Li W, Omidvar N, Kedjouar B, Turkson J et al. Stat3 controls lysosomal-mediated cell death in vivo. Nat Cell Biol 2011; 13: 303–309.
Yeung BHY, Huang D-C, Sinicrope FA . PS-341 (Bortezomib) induces lysosomal cathepsin B release and a caspase-2-dependent mitochondrial permeabilization and apoptosis in human pancreatic cancer cells. J Biol Chem 2006; 281: 11923–11932.
Bien S, Rimmbach C, Neumann H, Niessen J, Reimer E, Ritter CA et al. Doxorubicin-induced cell death requires cathepsin B in HeLa cells. Biochem Pharmacol 2010; 80: 1466–1477.
Taha TA, Kitatani K, Bielawski J, Cho W, Hannun YA, Obeid LM . Tumor necrosis factor induces the loss of sphingosine kinase-1 by a cathepsin B-dependent mechanism. J Biol Chem 2005; 280: 17196–17202.
Guicciardi ME, Deussing J, Miyoshi H, Bronk SF, Svingen PA, Peters C . Cathepsin B contributes to TNF-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J Clin Invest 2000; 106: 1127–1137.
Pratt MR, Sekedat MD, Chiang KP, Muir TW . Direct measurement of cathepsin B activity in the cytosol of apoptotic cells by an activity-based probe. Chem Biol 2009; 16: 1001–1012.
Droga-Mazovec G, Bojič L, Petelin A, Ivanova S, Romih R, Repnik U et al. Cysteine cathepsins trigger caspase-dependent cell death through cleavage of Bid and antiapoptotic Bcl-2 homologues. J Biol Chem 2008; 283: 19140–19150.
Johnston JB, Kabore AF, Strutinsky J, Hu X, Paul JT, Kropp DM et al. Role of the TRAIL//APO2-L death receptors in chlorambucil- and fludarabine-induced apoptosis in chronic lymphocytic leukemia. Oncogene 2003; 22: 8356–8369.
Riccardi C, Nicoletti I . Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc 2006; 1: 1458–1461.
Cheson BD, Bennett JM, Grever M, Kay N, Keating MJ, O'Brien S et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood 1996; 87: 4990–4997.
Lagace DC, Nachtigal MW . Inhibition of histone deacetylase activity by valproic acid blocks adipogenesis. J Biol Chem 2004; 279: 18851–18860.
El-Khoury V, Moussay E, Janji B, Palissot V, Aouali N, Brons NHC et al. The histone deacetylase inhibitor MGCD0103 induces apoptosis in B-cell chronic lymphocytic leukemia cells through a mitochondria-mediated caspase activation cascade. Mol Cancer Ther 2010; 9: 1349–1360.
Mizushima N, Klionsky DJ . Protein turnover via autophagy: implications for metabolism*. Annu Rev Nutr 2007; 27: 19–40.
Clague MJ, Urbé S . Ubiquitin: same molecule, different degradation pathways. Cell 2010; 143: 682–685.
Fischer U, Janicke RU, Schulze-Osthoff K . Many cuts to ruin: a comprehensive update of caspase substrates. Cell Death Differ 2003; 10: 76–100.
Berghe TV, Vanlangenakker N, Parthoens E, Deckers W, Devos M, Festjens N et al. Necroptosis, necrosis and secondary necrosis converge on similar cellular disintegration features. Cell Death Differ 2010; 17: 922–930.
Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute–Working Group 1996 guidelines. Blood 2008; 111: 5446–5456.
Vire B, David A, TOSO Wiestner A . The Fcμ receptor, is highly expressed on chronic lymphocytic leukemia B cells, internalizes upon IgM binding, shuttles to the lysosome, and is downregulated in response to tlr activation. J Immunol 2011; 187: 4040–4050.
Lim SH, Vaughan AT, Ashton-Key M, Williams EL, Dixon SV, Chan HTC et al. Fc gamma receptor IIb on target B cells promotes rituximab internalization and reduces clinical efficacy. Blood 2011; 118: 2530–2540.
Jak M, van Bochove GG, Reits EA, Kallemeijn WW, Tromp JM, Umana P et al. CD40 stimulation sensitizes CLL cells to lysosomal cell death induction by type II anti-CD20 monoclonal antibody GA101. Blood 2011; 118: 5178–5188.
Balakrishnan K, Burger JA, Quiroga MP, Henneberg M, Ayres ML, Wierda WG et al. Influence of bone marrow stromal microenvironment on forodesine-induced responses in CLL primary cells. Blood 2010; 116: 1083–1091.
Rudolf E, Červinka M . Sulforaphane induces cytotoxicity and lysosome- and mitochondria-dependent cell death in colon cancer cells with deleted p53. Toxicol In Vitro 2011; 25: 1302–1309.
Cheriyath V, Kuhns MA, Kalaycio ME, Borden EC . Potentiation of apoptosis by histone deacetylase inhibitors and doxorubicin combination: cytoplasmic cathepsin B as a mediator of apoptosis in multiple myeloma. Br J Cancer 2011; 104: 957–967.
Werneburg NW, Guicciardi ME, Bronk SF, Gores GJ . Tumor necrosis factor-alpha-associated lysosomal permeabilization is cathepsin B dependent. Am J Physiol Gastrointest Liver Physiol 2002; 283: G947–G956.
Meusers P, König E, Fink U, Brittinger G . Lysosomal acid hydrolases in isolated normal and chronic lymphocytic leukemia (CLL) T- and B-lymphocytes. Haematol Blood Transfus 1977; 20: 171–174.
Schmidt D, Radzun H, Schwarze E, Stein H, Parwaresch M . Activity and isoenzymes of acid phosphatase in human B-cell lymphomas of low-grade malignancy: a novel aid in the classification of malignant lymphoma. Cancer 1980; 46: 2676–2681.
Acknowledgements
We acknowledge the Manitoba Tumour Bank, especially Brenda Kuschak and Donna Hewitt for providing samples and Courtney Edworthy for clinical data. We thank all the patients who gave their blood samples to the Manitoba Tumour Bank and made this research possible. This study was supported by the Manitoba Tumour Bank, Winnipeg, Manitoba, funded in part by the CancerCare Manitoba Foundation and is a member of the Canadian Tumour Repository Network (CTRNet). This research was funded by the Leukemia and Lymphoma Society of Canada and CancerCare Manitoba Foundation. J-YY received a Studentship from Canadian Institutes of Health Research. SBG is funded by a Manitoba Health Research Chair.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Blood Cancer Journal website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Yoon, JY., Szwajcer, D., Ishdorj, G. et al. Synergistic apoptotic response between valproic acid and fludarabine in chronic lymphocytic leukaemia (CLL) cells involves the lysosomal protease cathepsin B. Blood Cancer Journal 3, e153 (2013). https://doi.org/10.1038/bcj.2013.50
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bcj.2013.50
Keywords
This article is cited by
-
Lysosomotropic agents selectively target chronic lymphocytic leukemia cells due to altered sphingolipid metabolism
Leukemia (2016)
-
Lysosomal ceramide generated by acid sphingomyelinase triggers cytosolic cathepsin B-mediated degradation of X-linked inhibitor of apoptosis protein in natural killer/T lymphoma cell apoptosis
Cell Death & Disease (2015)
-
Pharmacological Modulation of Photoreceptor Outer Segment Degradation in a Human iPS Cell Model of Inherited Macular Degeneration
Molecular Therapy (2015)
-
Valproic acid enhances fludarabine-induced apoptosis mediated by ROS and involving decreased AKT and ATM activation in B-cell-lymphoid neoplastic cells
Apoptosis (2014)