Abstract
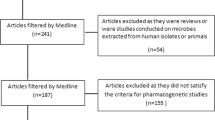
The decision taken by research ethics committees (RECs) while assessing pharmacogenetic (PGx) substudies as part of international clinical trials is almost unknown. A total of 255 applications of 36 PGx substudies embedded in clinical trials (12 phase 2, 24 phase 3) were submitted to 72 RECs in 2006–2007 by GlaxoSmithKline in Spain. These were trials of 17 different compounds, aimed to be conducted in the five continents. Of the 255 applications, 226 (89%) were directly approved by RECs without raising any queries to the sponsor; 1% (3/255) were plainly rejected by two RECs. The rest (10%) were followed by 64 queries asked by 16 RECs on 25 PGx substudies. Following responses from the sponsor, all but two applications were approved. Thus, the RECs involved finally approved 98% (250/255) of the submitted applications. The requirements specifically raised by two RECs (PGx samples to be transferred to a public biobank or alternatively destroyed immediately, or storage permitted only 5 years after the trial is concluded) could not be met by the sponsor. It can be inferred from the results obtained that ethical and scientific standards implemented by the sponsor in the design, conduct and sample management of PGx substudies satisfied the vast majority (70/72; 97%) of RECs involved in this study.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Relling MV, Hoffman JM . Should pharmacogenomic studies be required for new drug approval? Clin Pharmacol Ther 2007; 81: 425–428.
SACGHS Secretary's Advisory Committee on Genetics, Health, and Society. Realizing the Promise of Pharmacogenomics: Opportunities and Challenges, May 2008 [Accessed 20 August 2008]. Available at http://www4.od.nih.gov/oba/SACGHS/reports/SACGHS_PGx_Report.pdf.
Roses AD . Pharmacogenetics in drug discovery and development: a translational perspective. Nat Rev Drug Discov 2008; 7: 807–817.
Ingelman-Sundberg M . Pharmacogenomic biomarkers for prediction of severe adverse drug reactions. N Engl J Med 2008; 358: 637–639.
European Medicines Agency. Committee for Proprietary Medicinal Products (CPMP). Position paper on terminology in pharmacogenetics. EMEA/CPMP/3070/01. London, 21 November 2002. [Accessed on 18 August 2008]. Available at http://www.emea.europa.eu/pdfs/human/press/pp/307001en.pdf.
European Medicines Agency. ICH Topic E15. Definitions for genomic biomarkers, pharmacogenomics, pharmacogenetics, genomic data and sample coding categories. EMEA/CHMP/ICH/437986/2006. London, November 2007. [Accessed on 18 August 2008]. Available at http://www.emea.europa.eu/pdfs/human/ich/43798606en.pdf.
US Department of Health and Human Services. Food and Drug Administration. Guidance for industry. Pharmacogenomic data submissions. March 2005. [Accessed on 19 August 2008] Available at http://www.fda.gov/cder/guidance/6400fnl.pdf.
US Department of Health and Human Services. Food and Drug Administration. Guidance for industry and FDA staff. Pharmacogenetic tests and genetic tests for heritable markers. [Accessed on 2 September 2008] Available at http://www.fda.gov/cdrh/oivd/guidance/1549.pdf.
Council for International Organizations of Medical Sciences (CIOMS). Pharmacogenetics: towards improving treatments with medicines. 2005. Geneva, Switzerland. [Accessed on 2 September 2008] Available at http://www.cioms.ch/frame_pharmacogenetics_febr_2005.htm.
Nuffield Council on Bioethics. Pharmacogenetics: ethical issues. September 2003. [Accessed on 20 August 2008]. Available at http://www.nuffieldbioethics.org/fileLibrary/pdf/pharmacogenetics_report.pdf.
Rodríguez-Villanueva J, Alsar MJ, Avendaño C, Gómez-Piqueras C, Garcia-Alonso F . Pharmacogenetic studies: evaluation guidelines for research ethics committees. Scientific background and legal framework. (I). Med Clín (Barc) 2003; 120: 63–67, [in Spanish].
Rodríguez-Villanueva J, Alsar MJ, Avendaño C, Gómez-Piqueras C, Garcia-Alonso F . Pharmacogenetic studies: evaluation guidelines for research ethics committees. Study protocol and patient information sheet (II). Med Clin (Barc) 2003; 120: 101–107, [in Spanish].
Breckenridge A, Lindpaintner K, Lipton P, McLeod H, Rothstein M, Wallace H . Pharmacogenetics: ethical problems and solutions. Nat Rev Genet 2004; 5: 676–677.
Directive 2001/20/CE of the European Parliament and the Council of 4 April 2001 on the approximation of the laws, regulations and administrative provisions of the Member States relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use. Official J Eur Commun 2001; L121: 34–44.
Burman WJ, Reves RR, Cohn DL, Schooley RT . Breaking the camel's back: multicenter clinical trials and local institutional review boards. Ann Intern Med 2001; 134: 152–157.
Keinonen T, Nieminen S, Saareks V, Saano V, Ylitalo P . Acceptability and profile of the clinical drug trials underway in Finnish university hospitals in the 1990s: Applications reviewed by ethics committees. Methods Find Exp Clin Pharmacol 2001; 23: 415–423.
Dal-Ré R, Ortega R, Morejón E . Multicentre trials review process by research ethics committees in Spain: where do they stand before implementing the new European regulation? J Med Ethics 2005; 31: 744–750.
Angell E, Sutton AJ, Windridge K, Dixon-Woods M . Consistency in decision making by research ethics committees: a controlled comparison. J Med Ethics 2006; 32: 662–664.
Edwards SJ, Stone T, Swift T . Differences between research ethics committees. Int J Technol Assess Health Care 2007; 23: 17–23.
Lambers Heerspink H, Dobre D, Hillege HL, Grobbe DE, Zeeuw D, for the Collaborative Study Group. Does the European clinical trials directive really improve clinical trial approval time? Br J Clin Pharmacol 2008; 66: 546–550.
Arledge T, Freeman A, Arbuckle J, Mosteller M, Manasco P . Applications of phamacogenetics to drug development: the Glaxo Wellcome experience. Drug Metab Rev 2000; 32: 387–394.
Levine RJ . Ethics and Regulation of Clinical Research. 2nd ed. Urban & Schwarzenberg: Baltimore, 1986.
Council for International Organizations of Medical Sciences (CIOMS). International Ethical Guidelines for Biomedical Research Involving Human Subjects. Geneva, Switzerland., 2002.
Mallal S, Phillips E, Carosi G, Molina JM, Workman C, Tomazic J et al. PREDICT-1 Study Team. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med 2008; 358: 568–579.
Hughes AR, Spreen WR, Mosteller M, Warren LL, Lai EH, Brothers CH et al. Pharmacogenetics of hypersensitivity to abacavir: from PGx hypothesis to confirmation to clinical utility. Pharmacogenomics J 2008; 8: 365–374.
Hetherington S, Hughes AR, Mosteller M, Shortino D, Baker KL, Spreen W et al. Genetic variations in HLA-B region and hypersensitivity reactions to abacavir. Lancet 2002; 359: 1121–1122.
Rettie AE, Korzekwa KR, Kunze KL, Lawrence RF, Eddy AC, Aoyama T et al. Hydroxylation of warfarin by human cDNA-expressed cytochrome P-450: a role for P-4502C9 in the etiology of (S)-warfarin-drug interactions. Chem Res Toxicol 1992; 5: 54–59.
Anderson JL, Horne BD, Stevens SM, Grove AS, Barton S, Nicholas ZP et al. Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation. Circulation 2007; 116: 2563–2570.
Lesko LJ . The critical path of warfarin dosing: finding an optimal dosing strategy using pharmacogenetics. Clin Pharmacol Ther 2008; 84: 301–303.
Garcia DA . Warfarin and pharmacogenomic testing: the case for restraint. Clin Pharmacol Ther 2008; 84: 303–306.
Ley 14/2007 de 3 de julio, de Investigación Biomédica. BOE n° 159, de 4 de julio de 2007: 28826–28848.
Author information
Authors and Affiliations
Corresponding author
Additional information
Duality of interest
The authors work at GlaxoSmithKline SA, Tres Cantos, Madrid, Spain.
Rights and permissions
About this article
Cite this article
Dal-Ré, R., Luque, I., Torres, R. et al. Drug development: assessment of pharmacogenetic studies by Spanish research ethics committees. Pharmacogenomics J 9, 86–89 (2009). https://doi.org/10.1038/tpj.2008.18
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tpj.2008.18
Keywords
This article is cited by
-
Challenges in Obtaining Adequate Genetic Sample Sets in Clinical Trials: The Perspective of the Industry Pharmacogenomics Working Group
Clinical Pharmacology & Therapeutics (2011)