Abstract
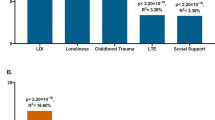
Allostatic load (AL) is the cumulative ‘wear and tear’ on the body due to chronic adversity. We tested the poly-environmental (exposomic) and polygenic contributions to AL and their combined contribution to adolescent mental health. In this cohort study of N = 5,036 diverse youth (mean age 12 years) from the Adolescent Brain Cognitive Development Study, we calculated a latent AL score, childhood exposomic risk and genetic risk. We tested the associations of exposomic and polygenic risks with AL using linear mixed-effects models, and tested the mediating role of AL on the pathway from exposomic/polygenic risk to mental health. AL was significantly lower among non-Hispanic white youth compared to Hispanic and non-Hispanic black youth. Childhood exposomic burden was associated with AL in adolescence (β = 0.25, 95% CI 0.22–0.29, P < 0.001). In subset analysis of participants of European-like genetic ancestry (n = 2,928), the type 2 diabetes polygenic risk score (T2D-PRS; β = 0.11, 95% CI 0.07–0.14, P < 0.001) and major depressive disorder (MDD)-PRS (β = 0.05, 95% CI 0.02–0.09, P = 0.003) were associated with AL. Both PRSs showed significant gene–environment interactions such that, with greater polygenic risk, associations between exposome and AL were stronger. AL significantly mediated the indirect path from exposomic risk at age 11 years, and from both MDD-PRS and T2D-PRS to psychopathology at age 12 years. Our findings show that AL can be quantified in youth and is associated with exposomic and polygenic burden, supporting the diathesis–stress model.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$59.00 per year
only $4.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Data used in the preparation of this Article were obtained from the ABCD Study (https://abcdstudy.org), held in the National Institute of Mental Health Data Archive and available for researchers upon application (https://nda.nih.gov/abcd/request-access).
Code availability
Code is available at https://github.com/barzilab1/ABCD_Allostatic_load.
References
Guidi, J., Lucente, M., Sonino, N. & Fava, G. A. Allostatic load and its impact on health: a systematic review. Psychother. Psychosom. 90, 11–27 (2021).
McEwen, B. S. Protective and damaging effects of stress mediators. New Engl. J. Med. 338, 171–179 (1998).
McEwen, B. S. & Wingfield, J. C. The concept of allostasis in biology and biomedicine. Horm. Behav. 43, 2–15 (2003).
McEwen, B. S. & Seeman, T. Protective and damaging effects of mediators of stress: elaborating and testing the concepts of allostasis and allostatic load. Ann. NY Acad. Sci. 896, 30–47 (1999).
McEwen, B. S. Stress, adaptation and disease: allostasis and allostatic load. Ann. NY Acad. Sci. 840, 33–44 (1998).
Seeman, T. E., McEwen, B. S., Rowe, J. W. & Singer, B. H. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc. Natl Acad. Sci. USA 98, 4770–4775 (2001).
Kapczinski, F. et al. Allostatic load in bipolar disorder: implications for pathophysiology and treatment. Neurosci. Biobehav. Rev. 32, 675–692 (2008).
Finlay, S., Rudd, D., McDermott, B. & Sarnyai, Z. Allostatic load in psychiatry—systematic review and meta-analysis. Psychoneuroendocrinology 131, 105555 (2021).
McEwen, B. S. Mood disorders and allostatic load. Biol. Psychiatry 54, 200–207 (2003).
Rogosch, F. A., Dackis, M. N. & Cicchetti, D. Child maltreatment and allostatic load: consequences for physical and mental health in children from low-income families. Dev. Psychopathol. 23, 1107–1124 (2011).
Berger, M. et al. Relationship between allostatic load and clinical outcomes in youth at ultra-high risk for psychosis in the NEURAPRO study. Schizophr. Res. 226, 38–43 (2020).
Brody, G. H., Lei, M.-K., Chen, E. & Miller, G. E. Neighborhood poverty and allostatic load in African American youth. Pediatrics 134, e1362–e1368 (2014).
Sabbah, W., Watt, R. G., Sheiham, A. & Tsakos, G. Effects of allostatic load on the social gradient in ischaemic heart disease and periodontal disease: evidence from the Third National Health and Nutrition Examination Survey. J. Epidemiol. Community Health 62, 415–420 (2008).
Stabellini, N. et al. Allostatic load and cardiovascular outcomes in males with prostate cancer. JNCI Cancer Spectr. 7, pkad005 (2023).
Lueth, A. J. et al. Allostatic load and adverse pregnancy outcomes. Obstet. Gynecol. 140, 974–982 (2022).
De Felice, F. G., Gonçalves, R. A. & Ferreira, S. T. Impaired insulin signalling and allostatic load in Alzheimer disease. Nat. Rev. Neurosci. 23, 215–230 (2022).
Gillespie, S. L. et al. Allostatic load in the association of depressive symptoms with incident coronary heart disease: the Jackson Heart Study. Psychoneuroendocrinology 109, 104369 (2019).
Nelson, S., Bento, S. & Enlow, M. B. Biomarkers of allostatic load as correlates of impairment in youth with chronic pain: an initial investigation. Children 8, 709 (2021).
Parker, H. W., Abreu, A. M., Sullivan, M. C. & Vadiveloo, M. K. Allostatic load and mortality: a systematic review and meta-analysis. Am. J. Prev. Med. 63, 131–140 (2022).
Beckie, T. M. A systematic review of allostatic load, health and health disparities. Biol. Res. Nurs. 14, 311–346 (2012).
Zheng, D. D. et al. Visual acuity and increased mortality: the role of allostatic load and functional status. Invest. Ophthalmol. Vis. Sci. 55, 5144–5150 (2014).
Bruun-Rasmussen, N. E. et al. Allostatic load as predictor of mortality: a cohort study from Lolland-Falster, Denmark. BMJ Open 12, e057136 (2022).
Duru, O. K., Harawa, N. T., Kermah, D. & Norris, K. C. Allostatic load burden and racial disparities in mortality. J. Natl Med. Assoc. 104, 89–95 (2012).
Szanton, S. L., Gill, J. M. & Allen, J. K. Allostatic load: a mechanism of socioeconomic health disparities? Biol. Res. Nurs. 7, 7–15 (2005).
Jeste, D. V. et al. Review of major social determinants of health in schizophrenia-spectrum psychotic disorders: III. Biology. Schizophr. Bull. 49, 867–880 (2023).
Danese, A. & McEwen, B. S. Adverse childhood experiences, allostasis, allostatic load and age-related disease. Physiol. Behav. 106, 29–39 (2012).
Fox, S. E., Levitt, P. & Nelson III, C. A. How the timing and quality of early experiences influence the development of brain architecture. Child Dev. 81, 28–40 (2010).
Hughes, K. et al. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public Health 2, e356–e366 (2017).
Shonkoff, J. P., Boyce, W. T. & McEwen, B. S. Neuroscience, molecular biology and the childhood roots of health disparities. JAMA 301, 2252–2259 (2009).
McCrory, C. et al. Towards a consensus definition of allostatic load: a multi-cohort, multi-system, multi-biomarker individual participant data (IPD) meta-analysis. Psychoneuroendocrinology 153, 106117 (2023).
Carbone, J. T., Clift, J. & Alexander, N. Measuring allostatic load: approaches and limitations to algorithm creation. J. Psychosom. Res. 163, 111050 (2022).
King, A. L., Garnier-Villarreal, M., Simanek, A. M. & Johnson, N. L. Testing allostatic load factor structures among adolescents: a structural equation modeling approach. Am. J. Hum. Biol. 31, e23242 (2019).
Doan, S. N. Allostatic load: developmental and conceptual considerations in a multi-system physiological indicator of chronic stress exposure. Dev. Psychobiol. 63, 825–836 (2021).
Li, J. et al. Association between early life adversity and allostatic load in girls with precocious puberty. Psychoneuroendocrinology 152, 106101 (2023).
Howard, J. T. & Sparks, P. J. Does allostatic load calculation method matter? Evaluation of different methods and individual biomarkers functioning by race/ethnicity and educational level. Am. J. Hum. Biol. 28, 627–635 (2016).
McEwen, B. S., & Stellar, E. Stress and the individual. Mechanisms leading to disease. Arch. Intern. Med. 153, 2093–2101 (1993).
Bobba-Alves, N., Juster, R.-P. & Picard, M. The energetic cost of allostasis and allostatic load. Psychoneuroendocrinology 146, 105951 (2022).
Loos, R. J. F. & Bouchard, C. Obesity—is it a genetic disorder? J. Intern. Med. 254, 401–425 (2003).
Qi, B., Liang, L., Doria, A., Hu, F. B. & Qi, L. Genetic predisposition to dyslipidemia and type 2 diabetes risk in two prospective cohorts. Diabetes 61, 745–752 (2012).
Petrovic, D. et al. Sociodemographic, behavioral and genetic determinants of allostatic load in a Swiss population-based study. Psychoneuroendocrinology 67, 76–85 (2016).
Schrempft, S. et al. Variation in the heritability of child body mass index by obesogenic home environment. JAMA Pediatr. 172, 1153–1160 (2018).
Wang, B. et al. Genetic contribution to the variance of blood pressure and heart rate: a systematic review and meta-regression of twin studies. Twin Res. Hum. Genet. 18, 158–170 (2015).
Simonis-Bik, A. M. C. et al. The heritability of HbA1c and fasting blood glucose in different measurement settings. Twin Res. Hum. Genet. 11, 597–602 (2008).
Heller, D. A., de Faire, U., Pedersen, N. L., Dahlen, G. & McClearn, G. E. Genetic and environmental influences on serum lipid levels in twins. N. Engl. J. Med. 328, 1150–1156 (1993).
Zhai, G. et al. Eight common genetic variants associated with serum DHEAS levels suggest a key role in ageing mechanisms. PLoS Genet. 7, e1002025 (2011).
Belsky, J. & Pluess, M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol. Bull. 135, 885–908 (2009).
Manuck, S. B. & McCaffery, J. M. Gene–environment interaction. Annu. Rev. Psychol. 65, 41–70 (2014).
Brody, G. H. et al. Cumulative socioeconomic status risk, allostatic load and adjustment: a prospective latent profile analysis with contextual and genetic protective factors. Dev. Psychol. 49, 913–927 (2013).
Brody, G. H. et al. Supportive family environments, genes that confer sensitivity, and allostatic load among rural African American emerging adults: a prospective analysis. J. Fam. Psychol. 27, 22–29 (2013).
Zheutlin, A. B. & Ross, D. A. Polygenic risk scores: what are they good for? Biol. Psychiatry 83, e51–e53 (2018).
Moore, T. M. et al. Modeling environment through a general exposome factor in two independent adolescent cohorts. Exposome 2, osac010 (2022).
Logan, A. C., Prescott, S. L., Haahtela, T. & Katz, D. L. The importance of the exposome and allostatic load in the planetary health paradigm. J. Physiol. Anthropol. 37, 15 (2018).
Whelan, E., O’Shea, J., Hunt, E. & Dockray, S. Evaluating measures of allostatic load in adolescents: a systematic review. Psychoneuroendocrinology 131, 105324 (2021).
McEwen, C. A. Connecting the biology of stress, allostatic load and epigenetics to social structures and processes. Neurobiol. Stress 17, 100426 (2022).
Deary, V. et al. Genetic contributions to self-reported tiredness. Mol. Psychiatry 23, 609–620 (2017).
Sacks, V. & Murphey, D. The Prevalence of Adverse Childhood Experiences, Nationally, by State, and by Race or Ethnicity. https://www.childtrends.org/publications/prevalence-adverse-childhood-experiences-nationally-state-race-ethnicity (Child Trends, 2018).
Kerr, P., Kheloui, S., Rossi, M., Désilets, M. & Juster, R.-P. Allostatic load and women’s brain health: a systematic review. Front. Neuroendocrinol. 59, 100858 (2020).
Hilliard, M. E. et al. Stress and A1c among people with diabetes across the lifespan. Curr. Diab. Rep. 16, 67 (2016).
Dutheil, F. et al. DHEA as a biomarker of stress: a systematic review and meta-analysis. Front. Psychiatry 12, 688367 (2021).
Reise, S. P., Moore, T. M. & Haviland, M. G. Bifactor models and rotations: exploring the extent to which multidimensional data yield univocal scale scores. J. Pers. Assess. 92, 544–559 (2010).
Bentler, P. M. & Bonett, D. G. Significance tests and goodness of fit in the analysis of covariance structures. Psychol. Bull. 88, 588–606 (1980).
Lucente, M. & Guidi, J. Allostatic load in children and adolescents: a systematic review. Psychother. Psychosom. 92, 295–303 (2023).
Borrell, L. N., Dallo, F. J. & Nguyen, N. Racial/ethnic disparities in all-cause mortality in US adults: the effect of allostatic load. Public Health Rep. 125, 810–816 (2010).
Geronimus, A. T. et al. Weathering in Detroit: place, race, ethnicity and poverty as conceptually fluctuating social constructs shaping variation in allostatic load. Milbank Q. 98, 1171–1218 (2020).
Moore, J. X., Bevel, M. S., Aslibekyan, S. & Akinyemiju, T. Temporal changes in allostatic load patterns by age, race/ethnicity and gender among the US adult population; 1988–2018. Prev. Med. 147, 106483 (2021).
Kristen Peek, M. et al. Allostatic load among non-hispanic whites, non-hispanic blacks and people of Mexican origin: effects of ethnicity, nativity and acculturation. Am. J. Public Health 100, 940–946 (2010).
Finlay, S. et al. Adverse childhood experiences and allostatic load: a systematic review. Neurosci. Biobehav. Rev. 136, 104605 (2022).
Atkinson, L. et al. Social engagement and allostatic load mediate between adverse childhood experiences and multimorbidity in mid to late adulthood: the Canadian Longitudinal Study on Aging. Psychol. Med. 53, 1437–1447 (2023).
Baccarelli, A., Dolinoy, D. C. & Walker, C. L. A precision environmental health approach to prevention of human disease. Nat. Commun. 14, 2449 (2023).
Rappaport, S. M. & Smith, M. T. Environment and disease risks. Science 330, 460–461 (2010).
Lueth, A. J. et al. Can allostatic load in pregnancy explain the association between race and subsequent cardiovascular disease risk: a cohort study. BJOG 130, 1197–1206 (2023).
Dunn, E. C. et al. Genetic determinants of depression: recent findings and future directions. Harv. Rev. Psychiatry 23, 1–18 (2015).
Warrier, V. et al. Gene–environment correlations and causal effects of childhood maltreatment on physical and mental health: a genetically informed approach. Lancet Psychiatry 8, 373–386 (2021).
Avshalom, C., Hariri, A. R., Andrew, H., Uher, R. & Moffitt, T. E. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am. J. Psychiatry 167, 509–527 (2010).
Zhao, M. et al. BDNF Val66Met polymorphism, life stress and depression: a meta-analysis of gene-environment interaction. J. Affect. Disord. 227, 226–235 (2018).
McAllister, K. et al. Current challenges and new opportunities for gene-environment interaction studies of complex diseases. Am. J. Epidemiol. 186, 753–761 (2017).
Lewis, C. M. & Vassos, E. Polygenic risk scores: from research tools to clinical instruments. Genome Med. 12, 44 (2020).
Van Heeringen, K. & Mann, J. J. The neurobiology of suicide. Lancet Psychiatry 1, 63–72 (2014).
Mann, J. The neurobiology of suicide. Nat. Med. 4, 25–30 (1998).
O’Shields, J., Mowbray, O. & Patel, D. Allostatic load as a mediator of childhood maltreatment and adulthood depressive symptoms: a longitudinal analysis. Psychoneuroendocrinology 143, 105839 (2022).
Scheuer, S. et al. Childhood abuse and depression in adulthood: the mediating role of allostatic load. Psychoneuroendocrinology 94, 134–142 (2018).
Ren, Y., Zuo, C., Ming, H., Zhang, Y. & Huang, S. Long-term neighborhood poverty effects on internalizing symptoms in adolescents: mediated through allostatic load and pubertal timing. J. Adolesc. Health https://doi.org/10.1016/J.JADOHEALTH.2023.08.027 (2023).
Prior, L., Manley, D. & Jones, K. Stressed out? An investigation of whether allostatic load mediates associations between neighbourhood deprivation and health. Health Place 52, 25–33 (2018).
John, A. P., Koloth, R., Dragovic, M. & Lim, S. C. B. Prevalence of metabolic syndrome among Australians with severe mental illness. Med. J. Aust. 190, 176–179 (2009).
Ducat, L., Philipson, L. H. & Anderson, B. J. The mental health comorbidities of diabetes. JAMA 312, 691–692 (2014).
Quek, Y. H., Tam, W. W. S., Zhang, M. W. B. & Ho, R. C. M. Exploring the association between childhood and adolescent obesity and depression: a meta-analysis. Obesity Rev. 18, 742–754 (2017).
Casagrande, S. et al. Increased glucocorticoid concentrations in early life cause mitochondrial inefficiency and short telomeres. J. Exp. Biol. 223, jeb222513 (2020).
Bobba-Alves, N. et al. Cellular allostatic load is linked to increased energy expenditure and accelerated biological aging. Psychoneuroendocrinology 155, 106322 (2023).
Picard, M., Juster, R. P. & McEwen, B. S. Mitochondrial allostatic load puts the ‘gluc’ back in glucocorticoids. Nat. Rev. Endocrinol. 10, 303–310 (2014).
Juster, R. P. et al. A transdisciplinary perspective of chronic stress in relation to psychopathology throughout life span development. Dev. Psychopathol. 23, 725–776 (2011).
Ellis, B. J. & Del Giudice, M. Beyond allostatic load: rethinking the role of stress in regulating human development. Dev. Psychopathol. 26, 1–20 (2014).
Callaghan, B. L. & Tottenham, N. The Stress Acceleration Hypothesis: effects of early-life adversity on emotion circuits and behavior. Curr. Opin. Behav. Sci. 7, 76–81 (2016).
Ehrlich, K. B., Yu, T., Sadiq, A. & Brody, G. H. Neighborhood poverty, allostatic load and changes in cellular aging in African American young adults: the moderating role of attachment. Attach. Hum. Dev. 24, 339–352 (2022).
McCrory, C. et al. How does socio-economic position (SEP) get biologically embedded? A comparison of allostatic load and the epigenetic clock(s). Psychoneuroendocrinology 104, 64–73 (2019).
Rosemberg, M. A. S., Granner, J., Li, Y. & Seng, J. S. A scoping review of interventions targeting allostatic load. Stress 23, 519–528 (2020).
Rebar, A. L. et al. A meta-meta-analysis of the effect of physical activity on depression and anxiety in non-clinical adult populations. Health Psychol. Rev. 9, 366–378 (2015).
Hu, M. X. et al. Exercise interventions for the prevention of depression: a systematic review of meta-analyses. BMC Public Health 20, 1255 (2020).
Joseph, J. The ‘missing heritability’ of psychiatric disorders: elusive genes or non-existent genes? Appl. Dev. Sci. 16, 65–83 (2012).
Baselmans, B. M. L., Yengo, L., van Rheenen, W. & Wray, N. R. Risk in relatives, heritability, SNP-based heritability and genetic correlations in psychiatric disorders: a review. Biol. Psychiatry 89, 11–19 (2021).
Floriou-Servou, A. et al. The acute stress response in the multiomic era. Biol. Psychiatry 89, 1116–1126 (2021).
van Calker, D. & Serchov, T. The ‘missing heritability’—problem in psychiatry: is the interaction of genetics, epigenetics and transposable elements a potential solution? Neurosci. Biobehav. Rev. 126, 23–42 (2021).
Duncan, L. et al. Analysis of polygenic risk score usage and performance in diverse human populations. Nat. Commun. 10, 3328 (2019).
Yajurvedi, H. Stress and glucose metabolism: a review. Imaging J. Clin. Med. Sci. 5, 8–12 (2018).
Garavan, H. et al. Recruiting the ABCD sample: design considerations and procedures. Dev. Cogn. Neurosci. 32, 16–22 (2018).
Pries, L.-K. et al. Estimating the association between exposome and psychosis as well as general psychopathology: results from the ABCD study. Biol. Psychiatry Glob. Open Sci. 2, 283–291 (2022).
Schultz, L. M. et al. Stability of polygenic scores across discovery genome-wide association studies. Hum. Genet. Genom. Adv. 3, 100091 (2022).
Daskalakis, N. P. et al. Contributions of PTSD polygenic risk and environmental stress to suicidality in preadolescents. Neurobiol. Stress 15, 100411 (2021).
Chen, J. et al. Genome-wide association study of type 2 diabetes in Africa. Diabetologia 62, 1204–1211 (2019).
Scott, R. A. et al. An expanded genome-wide association study of type 2 diabetes in Europeans. Diabetes 66, 2888–2902 (2017).
Howard, D. M. et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat. Neurosci. 22, 343–352 (2019).
Levey, D. F. et al. Bi-ancestral depression GWAS in the Million Veteran Program and meta-analysis in >1.2 million individuals highlight new therapeutic directions. Nat. Neurosci. 24, 954–963 (2021).
Ge, T., Chen, C.-Y., Ni, Y., Feng, Y.-C. A. & Smoller, J. W. Polygenic prediction via Bayesian regression and continuous shrinkage priors. Nat. Commun. 10, 1776 (2019).
Locke, A. E. et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 518, 197–206 (2015).
Nievergelt, C. M. et al. International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nat. Commun. 10, 4558 (2019).
Schwarz, G. Estimating the dimension of a model. Ann. Stat 6, 461–464 (1978).
Reise, S. P. The rediscovery of bifactor measurement models. Multivariate Behav. Res. 47, 667–696 (2012).
Achenbach, T. M., McConaughy, S. H., Ivanova, M. Y. & Rescorla, L. A. Manual for the ASEBA Brief Problem Monitor (BPM) (ASEBA, 2011).
Achenbach, T. M. & Ruffle, T. M. The child behavior checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatr. Rev. 21, 265–271 (2000).
Nakagawa, S., Johnson, P. C. D. & Schielzeth, H. The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J. R. Soc. Interface 14, 20170213 (2017).
Rosseel, Y. lavaan: an R package for structural equation modeling. J. Stat. Softw. 48, 1–36 (2012).
Acknowledgements
Data used in the preparation of this article were obtained from the ABCD Study (https://abcdstudy.org), held in the NDA. This is a multisite, longitudinal study designed to recruit more than 10,000 children age 9–10 and follow them over 10 years into early adulthood. The ABCD Study is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041048, U01DA050989, U01DA051016, U01DA041022, U01DA051018, U01DA051037, U01DA050987, U01DA041174, U01DA041106, U01DA041117, U01DA041028, U01DA041134, U01DA050988, U01DA051039, U01DA041156, U01DA041025, U01DA041120, U01DA051038, U01DA041148, U01DA041093, U01DA041089, U24DA041123 and U24DA041147. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/consortium_members/. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in the analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the National Institutes of Health or ABCD consortium investigators. R.B. is supported by the National Institute of Mental Health (K23MH120437). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. A draft of this manuscript has been posted as a preprint on medRxiv (https://doi.org/10.1101/2023.10.27.23297674v1).
Author information
Authors and Affiliations
Contributions
K.W.H., T.M.M., M.M.G., O.K. and R.B. conceived and designed the study. K.T.T., E.V., G.E.D. and L.M.S. curated and organized the data. K.T.T., T.M.M., E.V. and L.M.S. analyzed the data. B.H.C., L.A., M.R.H. and N.P.D. substantially contributed to interpretation of the data. K.W.H., K.T.T. and R.B. wrote the first draft of the manuscript. M.M.G., O.K., B.H.C., L.M.S., L.A., M.R.H. and N.P.D. substantially revised the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
R.B. serves on the Scientific Advisory Board and holds equity in Taliaz Health, with no relevance to this work. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Mental Health thanks Zhiyang Wang and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–8 and Fig. 1.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hoffman, K.W., Tran, K.T., Moore, T.M. et al. Exposomic and polygenic contributions to allostatic load in early adolescence. Nat. Mental Health (2024). https://doi.org/10.1038/s44220-024-00255-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44220-024-00255-9