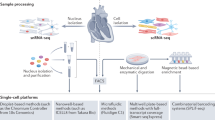
Abstract
Single-cell technology has become an indispensable tool in cardiovascular research since its first introduction in 2009. Here, we highlight the recent remarkable progress in using single-cell technology to study transcriptomic and epigenetic heterogeneity in cardiac disease and development. We then introduce the key concepts in single-cell multi-omics modalities that apply to cardiovascular research. Lastly, we discuss some of the trending concepts in single-cell technology that are expected to propel cardiovascular research to the next phase of single-cell research.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Shapiro, E., Biezuner, T. & Linnarsson, S. Single-cell sequencing-based technologies will revolutionize whole-organism science. Nat. Rev. Genet. 14, 618–630 (2013).
Tang, F. et al. mRNA-Seq whole-transcriptome analysis of a single cell. Nat. Methods 6, 377–382 (2009).
Cao, J. et al. The single-cell transcriptional landscape of mammalian organogenesis. Nature 566, 496–502 (2019).
Buettner, F. et al. Computational analysis of cell-to-cell heterogeneity in single-cell RNA-sequencing data reveals hidden subpopulations of cells. Nat. Biotechnol. 33, 155–160 (2015).
Domcke, S. et al. A human cell atlas of fetal chromatin accessibility. Science 370, eaba7612 (2020).
Turner, A. W. et al. Single-nucleus chromatin accessibility profiling highlights regulatory mechanisms of coronary artery disease risk. Nat. Genet. 54, 804–816 (2022).
Örd, T. et al. Single-cell epigenomics and functional fine-mapping of atherosclerosis GWAS loci. Circ. Res. https://doi.org/10.1161/CIRCRESAHA.121.318971 (2021).
Asp, M. et al. A spatiotemporal organ-wide gene expression and cell atlas of the developing human heart. Cell 179, 1647–1660 (2019). Spatiotemporal single-cell analyses of human embryo hearts, identifying distinctive gene profiles that correspond to discrete anatomical areas in each developmental stage, and providing a web-based resource of the developing human heart.
Stuart, T. & Satija, R. Integrative single-cell analysis. Nat. Rev. Genet. 20, 257–272 (2019)
Wang, Z., Gerstein, M. & Snyder, M. RNA-seq: a revolutionary tool for transcriptomics. Nat. Rev. Genet. 10, 57–63 (2009).
Paik, D. T., Cho, S., Tian, L., Chang, H. Y. & Wu, J. C. Single-cell RNA sequencing in cardiovascular development, disease and medicine. Nat. Rev. Cardiol. 17, 457–473 (2020).
Litviňuková, M. et al. Cells of the adult human heart. Nature 588, 466–472 (2020). Single-cell RNA-seq of an adult human heart reveals the distinct atrial and ventricular subsets of cells, highlighting the cellular heterogeneity of the human heart and providing an online human cardiac cell atlas for a valuable reference.
Chaffin, M. et al. Single-nucleus profiling of human dilated and hypertrophic cardiomyopathy. Nature 608, 174–180 (2022).
Tucker, N. R. et al. Transcriptional and cellular diversity of the human heart. Circulation 142, 466–482 (2020).
Koenig, A. L. et al. Single-cell transcriptomics reveals cell-type-specific diversification in human heart failure. Nat. Cardiovasc. Res. 1, 263–280 (2022).
Nicin, L. et al. A human cell atlas of the pressure-induced hypertrophic heart. Nat. Cardiovasc. Res. 1, 174–185 (2022).
Nicin, L. et al. Single-nuclei sequencing reveals novel insights into the regulation of cellular signatures in children with dilated cardiomyopathy. Circulation https://doi.org/10.1161/CIRCULATIONAHA.120.051391 (2021).
Kuppe, C. et al. Spatial multi-omic map of human myocardial infarction. Nature 608, 766–777 (2022). Spatial multi-omics analyses of human myocardial infarction tissue produced an integrative map of cardiac cell-type compositions at high-resolution, revealing changes in the cardiac transcriptome and epigenome by identifying distinct cardiac tissue structures during injury, repair, and remodeling
Reichart, D. et al. Pathogenic variants damage cell composition and single-cell transcription in cardiomyopathies. Science 377, eabo1984 (2022).
Hocker, J. D. et al. Cardiac cell-type-specific gene regulatory programs and disease risk association. Sci. Adv. 7, eabf1444 (2021).
Svensson, V., Vento-Tormo, R. & Teichmann, S. A. Exponential scaling of single-cell RNA-seq in the past decade. Nat. Protoc. 13, 599–604 (2018).
Wu, A. R. et al. Quantitative assessment of single-cell RNA-sequencing methods. Nat. Meth. 11, 41–46 (2014).
Buenrostro, J. D. et al. Single-cell chromatin accessibility reveals principles of regulatory variation. Nature 523, 486–490 (2015).
Lombardo, J. A., Aliaghaei, M., Nguyen, Q. H., Kessenbrock, K. & Haun, J. B. Microfluidic platform accelerates tissue processing into single cells for molecular analysis and primary culture models. Nat. Commun. 12, 2858 (2021).
Denisenko, E. et al. Systematic assessment of tissue dissociation and storage biases in single-cell and single-nucleus RNA-seq workflows. Genome Biol. 21, 130 (2020).
Bakken, T. E. et al. Single-nucleus and single-cell transcriptomes compared in matched cortical cell types. PLoS ONE 13, e209648 (2018).
Rosenberg, A. B. et al. Single-cell profiling of the developing mouse brain and spinal cord with split-pool barcoding. Science 360, 176–182 (2018).
Cao, J. et al. Comprehensive single-cell transcriptional profiling of a multicellular organism. Science 357, 661–667 (2017). Sci-RNA-seq, a combinatorial indexing method for profiling the transcriptomes of single cells or nuclei, was developed and profiled over 50,000 cells from the L2 larval stage of Caenorhabditis elegans, resulting in >50-fold ‘shotgun’ coverage of its somatic cell composition.
Cusanovich, D. A. et al. Multiplex single-cell profiling of chromatin accessibility by combinatorial cellular indexing. Science 348, 910–914 (2015).
Li, H. & Humphreys, B. D. Single-cell technologies: beyond microfluidics. Kidney360 2, 1196–1204 (2021).
Lareau, C. A. et al. Droplet-based combinatorial indexing for massive-scale single-cell chromatin accessibility. Nat. Biotechnol. 37, 916–924 (2019).
Ma, S. et al. Chromatin potential identified by shared single-cell profiling of RNA and chromatin. Cell 183, 1103–1116.e20 (2020).
Greener, J. G., Kandathil, S. M., Moffat, L. & Jones, D. T. A guide to machine learning for biologists. Nat. Rev. Mol. Cell Biol. 23, 40–55 (2022).
Moulik, M. et al. ANKRD1, the gene encoding cardiac ankyrin repeat protein, is a novel dilated cardiomyopathy gene. J. Am. Coll. Cardiol. 54, 325–333 (2009).
Wang, F. et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J. Mol. Diagnostics 14, 22–29 (2012).
Alexanian, M. et al. A transcriptional switch governs fibroblast activation in heart disease. Nature 595, 438–443 (2021).
Orchard, S. et al. The MIntAct project–IntAct as a common curation platform for 11 molecular interaction databases. Nucleic Acids Res. 42, D358–D363 (2014).
Jin, S. et al. Inference and analysis of cell–cell communication using CellChat. Nat. Commun. 12, 1088 (2021). CellChat is a tool that can statistically predict and analyze intercellular communication networks using single-cell RNA-sequencing data and thereby assist in the discovery of novel intercellular communications in various tissues.
Efremova, M., Vento-Tormo, M., Teichmann, S. A. & Vento-Tormo, R. CellPhoneDB: inferring cell–cell communication from combined expression of multi-subunit ligand–receptor complexes. Nat. Protoc. 15, 1484–1506 (2020).
Cabello-Aguilar, S. et al. SingleCellSignalR: inference of intercellular networks from single-cell transcriptomics. Nucleic Acids Res. 48, e55 (2021).
Gerety, S. S. & Anderson, D. J. Cardiovascular ephrinB2 function is essential for embryonic angiogenesis. Development 129, 1397–1410 (2002).
Saelens, W., Cannoodt, R., Todorov, H. & Saeys, Y. A comparison of single-cell trajectory inference methods. Nat. Biotechnol. 37, 547–554 (2019).
Bais, A. S. & Kostka, D. scds: computational annotation of doublets in single-cell RNA-sequencing data. Bioinformatics 36, 1150–1158 (2020).
DePasquale, E. A. K. et al. Doubletdecon: deconvoluting doublets from single-cell RNA-sequencing data. Cell Rep. 29, 1718–1727 (2019).
McGinnis, C. S., Murrow, L. M. & Gartner, Z. J. DoubletFinder: doublet detection in single-cell RNA-sequencing data using artificial nearest neighbors. Cell Syst. 8, 329–337 (2019).
Young, M. D. & Behjati, S. SoupX removes ambient RNA contamination from droplet-based single-cell RNA-sequencing data. Gigascience 9, giaa151 (2020).
Yang, S. et al. Decontamination of ambient RNA in single-cell RNA-seq with DecontX. Genome Biol. 21, 57 (2020).
Wang, X., He, Y., Zhang, Q., Ren, X. & Zhang, Z. Direct comparative analyses of 10x genomics chromium and Smart-seq2. Genomics Proteomics Bioinformatics 19, 253–266 (2021).
Osorio, D. & Cai, J. J. Systematic determination of the mitochondrial proportion in human and mice tissues for single-cell RNA-sequencing data quality control. Bioinformatics 37, 963–967 (2021).
Maniatis, T. Mechanisms of alternative pre-mRNA splicing. Science 251, 33–34 (1991).
Khan, M. A. F. et al. RBM20 regulates circular RNA production from the titin gene. Circ. Res. 119, 996–1003 (2016).
van den Hoogenhof, M. M. G. et al. RBM20 mutations induce an arrhythmogenic dilated cardiomyopathy related to disturbed calcium handling. Circulation 138, 1330–1342 (2018).
Harvey, S. E. & Cheng, C. Methods for characterization of alternative RNA splicing. in Long Non-coding RNAs: Methods and Protocols Vol. 1402 (eds. Feng, Y. & Zhang, L.) 229–241 (Humana Press, NY, 2016).
Volden, R. & Vollmers, C. Single-cell isoform analysis in human immune cells. Genome Biol. 23, 47 (2022).
Wong, E. S. et al. Deep conservation of the enhancer regulatory code in animals. Science 370, eaax8137 (2020).
Wirka, R. C. et al. Atheroprotective roles of smooth muscle cell phenotypic modulation and the TCF21 disease gene as revealed by single-cell analysis. Nat. Med. 25, 1280–1289 (2019).
Pliner, H. A. et al. Cicero predicts cis-regulatory DNA interactions from single-cell chromatin accessibility data. Mol. Cell 71, 858–871 (2018).
Tan, W. L. W. et al. Epigenomes of human hearts reveal new genetic variants relevant for cardiac disease and phenotype. Circ. Res. 761–777 https://doi.org/10.1161/CIRCRESAHA.120.317254 (2020).
Fulco, C. P. et al. Activity-by-contact model of enhancer–promoter regulation from thousands of CRISPR perturbations. Nat. Genet. 51, 1664–1669 (2019).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587 (2021). This study used simultaneous assessment of various modalities to define cellular states based on multimodal data, which improves our ability to identify previously unreported subpopulations, such as lymphoid.
Cao, J. et al. Joint profiling of chromatin accessibility and gene expression in thousands of single cells. Science 361, 1380–1385 (2018).
Zhu, C. et al. An ultra high-throughput method for single-cell joint analysis of open chromatin and transcriptome. Nat. Struct. Mol. Biol. 26, 1063–1070 (2019).
Pan, Q., Shai, O., Lee, L. J., Frey, B. J. & Blencowe, B. J. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat. Genet. 40, 1413–1415 (2008).
Emmerich, C. H. et al. Improving target assessment in biomedical research: the GOT-IT recommendations. Nat. Rev. Drug Disco. 20, 64–81 (2021).
Assarsson, E. et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS ONE 9, e95192 (2014).
Frei, A. P. et al. Highly multiplexed simultaneous detection of RNAs and proteins in single cells. Nat. Methods 13, 269–275 (2016).
Peterson, V. M. et al. Multiplexed quantification of proteins and transcripts in single cells. Nat. Biotechnol. 35, 936–939 (2017). REAP-seq is a technique that uses DNA-labeled antibodies and droplet microfluidics to assess the levels of gene and protein expression in single cells to identify and describe an unidentified cell type.
Stoeckius, M. et al. Simultaneous epitope and transcriptome measurement in single cells. Nat. Methods 14, 865–868 (2017).
Chung, H. et al. Joint single-cell measurements of nuclear proteins and RNA in vivo. Nat. Methods 18, 1204–1212 (2021).
Chen, A. F. et al. NEAT-seq: simultaneous profiling of intra-nuclear proteins, chromatin accessibility and gene expression in single cells. Nat. Methods https://doi.org/10.1038/s41592-022-01461-y (2022).
Longo, S. K., Guo, M. G., Ji, A. L. & Khavari, P. A. Integrating single-cell and spatial transcriptomics to elucidate intercellular tissue dynamics. Nat. Rev. Genet. 22, 627–644 (2021).
Crosetto, N., Bienko, M. & van Oudenaarden, A. Spatially resolved transcriptomics and beyond. Nat. Rev. Genet. 16, 57–66 (2015).
Chen, K. H., Boettiger, A. N., Moffitt, J. R., Wang, S. & Zhuang, X. RNA imaging. Spatially resolved, highly multiplexed RNA profiling in single cells. Science 348, aaa6090 (2015).
Eng, C. H. L. et al. Transcriptome-scale super-resolved imaging in tissues by RNA-seq FISH. Nature 568, 235–239 (2019).
Liu, Y. et al. High-spatial-resolution multi-omics sequencing via deterministic barcoding in tissue. Cell 183, 1665–1681 (2020).
Cho, C. S. et al. Microscopic examination of spatial transcriptome using Seq-Scope. Cell 184, 3559–3572 (2021). Seq-Scope, a spatial barcoding technology, outperforms existing methods by several orders of magnitude by enabling researchers to observe every gene expressed, as well as single cells and structures inside those cells, with an astonishingly high resolution of 0.6 μm.
Rodriques, S. G. et al. Slide-seq: a scalable technology for measuring genome-wide expression at high spatial resolution. Science 363, 1463–1467 (2019).
Resolve Biosciences. Key Findings. https://resolvebiosciences.com/ (2023).
Võsa, U. et al. Large-scale cis- and trans-eQTL analyses identify thousands of genetic loci and polygenic scores that regulate blood gene expression. Nat. Genet. 53, 1300–1310 (2021).
van der Wijst, M. G. P. et al. Single-cell RNA sequencing identifies cell-type-specific cis-eQTLs and coexpression QTLs. Nat. Genet. 50, 493–497 (2018). This study used single-cell eQTL analysis to uncover genetic variations that influence regulatory networks by producing expression profiles of 25,000 PBMCs from 45 donors and identifying previously described cis-eQTLs, as well as new cell-type-specific cis-eQTLs.
Elorbany, R. et al. Single-cell sequencing reveals lineage-specific dynamic genetic regulation of gene expression during human cardiomyocyte differentiation. PLoS Genet. 18, e1009666 (2022).
Neavin, D. et al. Single cell eQTL analysis identifies cell-type-specific genetic control of gene expression in fibroblasts and reprogrammed induced pluripotent stem cells. Genome Biol 22, 76 (2021).
Kang, H. M. et al. Multiplexed droplet single-cell RNA-sequencing using natural genetic variation. Nat. Biotechnol. 36, 89–94 (2018).
Cuomo, A. S. E. et al. Single-cell RNA-sequencing of differentiating iPS cells reveals dynamic genetic effects on gene expression. Nat. Commun. 11, 810 (2020).
Yazar, S. et al. Single-cell eQTL mapping identifies cell-type-specific genetic control of autoimmune disease. Science 376, eabf3041 (2022).
Mandric, I. et al. Optimized design of single-cell RNA-sequencing experiments for cell-type-specific eQTL analysis. Nat. Commun. 11, 5504 (2020).
Becht, E. et al. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. 37, 38–47 (2019).
Tran, D. et al. Fast and precise single-cell data analysis using a hierarchical autoencoder. Nat. Commun. 12, 1029 (2021).
Svensson, V., Gayoso, A., Yosef, N. & Pachter, L. Interpretable factor models of single-cell RNA-seq via variational autoencoders. Bioinformatics 36, 3418–3421 (2020).
Grønbech, C. H. et al. ScVAE: variational auto-encoders for single-cell gene expression data. Bioinformatics 36, 4415–4422 (2020).
Dong, G., Liao, G., Liu, H. & Kuang, G. A review of the autoencoder and its variants: a comparative perspective from target recognition in synthetic-aperture radar images. IEEE Geosci. Remote Sens. Mag. 6, 44–68 (2018)
Ding, J., Condon, A. & Shah, S. P. Interpretable dimensionality reduction of single cell transcriptome data with deep generative models. Nat. Commun. 9, 2002 (2018).
Wang, D. & Gu, J. VASC: dimension reduction and visualization of single-cell RNA-seq data by deep variational autoencoder. Genomics Proteomics Bioinformatics 16, 320–331 (2018).
Eraslan, G., Simon, L. M., Mircea, M., Mueller, N. S. & Theis, F. J. Single-cell RNA-seq denoising using a deep count autoencoder. Nat. Commun. 10, 390 (2019).
Amodio, M. et al. Exploring single-cell data with deep multitasking neural networks. Nat. Methods 16, 1139–1145 (2019).
Wang, T. et al. BERMUDA: a novel deep transfer learning method for single-cell RNA-sequencing batch correction reveals hidden high-resolution cellular subtypes. Genome Biol. 20, 165 (2019).
Liu, J. et al. Jointly defining cell types from multiple single-cell datasets using LIGER. Nat. Protoc. 15, 3632–3662 (2020).
Zeng, W. et al. DC3 is a method for deconvolution and coupled clustering from bulk and single-cell genomics data. Nat. Commun. 10, 4613 (2019).
Demetci, P., Santorella, R., Sandstede, B., Stafford Noble, W. & Singh, R. SCOT: single-cell multi-omics alignment with optimal transport. J. Comput. Biol. 29, 3–18 (2020).
Schiebinger, G. et al. Optimal-transport analysis of single-cell gene expression identifies developmental trajectories in reprogramming. Cell 176, 928–943 (2019). This paper introduced Waddington-OT, a method for analyzing developmental time courses to model the regulatory systems that underpin them and deduce ancestor-descendant outcomes showing a broader variety of developmental programs than those previously documented, including cell adaptability in either a terminal stromal state or a mesenchymal-to-epithelial transition state.
Bock, C. et al. High-content CRISPR screening. Nat. Rev. Meth. Prim. 2, 8 (2022).
VanDusen, N. J. et al. Massively parallel in vivo CRISPR screening identifies RNF20/40 as epigenetic regulators of cardiomyocyte maturation. Nat. Commun. 12, 4442 (2021).
Nishiga, M., Liu, C., Qi, L. S. & Wu, J. C. The use of new CRISPR tools in cardiovascular research and medicine. Nat. Rev. Cardiol. 19, 505–521 (2022)
Schraivogel, D. et al. Targeted Perturb-seq enables genome-scale genetic screens in single cells. Nat. Methods 17, 629–635 (2020).
Dixit, A. et al. Perturb-seq: dissecting molecular circuits with scalable single-cell RNA profiling of pooled genetic screens. Cell 167, 1853–1866 (2016).
Datlinger, P. et al. Pooled CRISPR screening with single-cell transcriptome readout. Nat. Methods 14, 297–301 (2017).
Cao, J. et al. A human cell atlas of fetal gene expression. Science 370, eaba7721 (2020). This study generated comprehensive atlases of early human development from two studies by Cao et al. and Domcke et al., using a pooled approach with three levels of combinatorial indexing, and examined the single-cell gene expression and chromatin landscapes of 15 organs in fetal samples.
Wang, L. et al. Single-cell reconstruction of the adult human heart during heart failure and recovery reveals the cellular landscape underlying cardiac function. Nat. Cell Biol. 22, 108–119 (2020).
Barruet, E. et al. Functionally heterogeneous human satellite cells identified by single-cell RNA sequencing. Elife 9, e51576 (2020).
Paik, D. T. et al. Large-scale single-cell RNA-seq reveals molecular signatures of heterogeneous populations of human induced pluripotent stem cell-derived endothelial cells. Circ. Res. 123, 443–450 (2018).
Li, Y. et al. Single-cell transcriptome analysis reveals dynamic cell populations and differential gene expression patterns in control and aneurysmal human aortic tissue. Circulation https://doi.org/10.1161/CIRCULATIONAHA.120.046528 (2020).
Pan, H. et al. Single-cell genomics reveals a novel cell state during smooth muscle cell phenotypic switching and potential therapeutic targets for atherosclerosis in mouse and human. Circulation 142, 2060–2075 (2020).
Selewa, A. et al. Systematic comparison of high-throughput single-cell and single-nucleus transcriptomes during cardiomyocyte differentiation. Sci Rep. 10, 1535 (2020).
Depuydt, M. A. C. et al. Microanatomy of the human atherosclerotic plaque by single-cell transcriptomics. Circ. Res. https://doi.org/10.1161/CIRCRESAHA.120.316770 (2020).
Cui, Y. et al. Single-cell transcriptome analysis maps the developmental track of the human heart. Cell Rep. 26, 1934–1950 (2019).
Nomura, S. et al. Cardiomyocyte gene programs encoding morphological and functional signatures in cardiac hypertrophy and failure. Nat. Commun. 9, 4435 (2018).
Churko J. M. et al. Defining human cardiac transcription factor hierarchies using integrated single-cell heterogeneity analysis. Nat. Commun. 9. 4906 (2018).
Wolf, F. A., Angerer, P. & Theis, F. J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 19, 15 (2018).
Acknowledgements
We acknowledge funding support from American Heart Association grant 20POST35210896 (to W.L.W.T.); F30 HL156478 (to A.Z.); National Institutes of Health (NIH) grants R01 HG010359 (to W.H.W.), and R01 HL145676, R01 HL146690, R01 HL130020, R01 HL145676, R01 HL141371 and R01HL126527 (to J.C.W.); and Chan Zuckerberg Initiative 2020-217708 and 2021-240451 (to J.C.W.). We thank B. C. Wu (Stanford University), D. T. Paik (Stanford University), S. S. Hye (Stanford University) and L. C. J. Mick (National University of Singapore) for critical reading of the manuscript.
Author information
Authors and Affiliations
Contributions
W.L.W.T. conceived and wrote the manuscript and created the figures. W.O.S., A.Z. and S.R. compiled the references and wrote the manuscript. W.H.W. and W.J.G. wrote the manuscript, with input from all authors. J.C.W. directed the manuscript and reviewed it for important intellectual content.
Corresponding author
Ethics declarations
Competing interests
W.J.G. is an equity holder of 10x Genomics and a cofounder of Protillion Biosciences and J.C.W. is a cofounder and scientific advisory board member for Greenstone Biosciences, but the work presented here is completely independent. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Cardiovascular Research thanks Kory Lavine, Minna Kaikkonen and Nathan Salomonis for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tan, W.L.W., Seow, W.Q., Zhang, A. et al. Current and future perspectives of single-cell multi-omics technologies in cardiovascular research. Nat Cardiovasc Res 2, 20–34 (2023). https://doi.org/10.1038/s44161-022-00205-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44161-022-00205-7
This article is cited by
-
A Multimodal Omics Framework to Empower Target Discovery for Cardiovascular Regeneration
Cardiovascular Drugs and Therapy (2024)