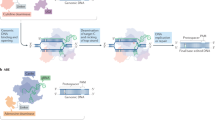
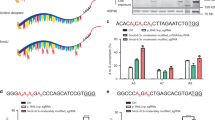
Abstract
Clustered regularly interspaced short palindromic repeats (CRISPR)-based genome editors are powerful tools in biology and hold great promise for the treatment of human diseases. Advanced DNA base editing tools, such as cytosine base editor and adenine base editor, have been developed to correct permanent mistakes in genetic material. However, undesired off-target edits would also be permanent, which poses a considerable risk for therapeutics. Alternatively, base editing at the RNA level is capable of correcting disease-causing mutations but does not lead to lasting genotoxic effects. RNA base editors offer temporary and reversible therapies and have been catching on in recent years. Here, we summarize some emerging RNA editors based on A-to-inosine, C-to-U and U-to-pseudouridine changes. We review the programmable RNA-targeting systems as well as modification enzyme-based effector proteins and highlight recent technological breakthroughs. Finally, we compare editing tools, discuss limitations and opportunities, and provide insights for the future directions of RNA base editing.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Landrum, M. J. et al. ClinVar: public archive of interpretations of clinically relevant variants. Nucleic Acids Res. 44, D862–D868 (2016).
Van Alstyne, M. et al. Gain of toxic function by long-term AAV9-mediated SMN overexpression in the sensorimotor circuit. Nat. Neurosci. 24, 930–940 (2021).
Doudna, J. A. The promise and challenge of therapeutic genome editing. Nature 578, 229–236 (2020).
Rees, H. A. & Liu, D. R. Base editing: precision chemistry on the genome and transcriptome of living cells. Nat. Rev. Genet. 19, 770–788 (2018).
Komor, A. C., Kim, Y. B., Packer, M. S., Zuris, J. A. & Liu, D. R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533, 420–424 (2016).
Gaudelli, N. M. et al. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature 551, 464–471 (2017).
Woolf, T. M., Chase, J. M. & Stinchcomb, D. T. Toward the therapeutic editing of mutated RNA sequences. Proc. Natl Acad. Sci. USA 92, 8298–8302 (1995).
Wang, X. et al. Develop a compact RNA base editor by fusing ADAR with engineered EcCas6e. Adv. Sci. 10, e2206813 (2023).
O’Connell, M. R. et al. Programmable RNA recognition and cleavage by CRISPR/Cas9. Nature 516, 263–266 (2014).
Marina, R. J., Brannan, K. W., Dong, K. D., Yee, B. A. & Yeo, G. W. Evaluation of engineered CRISPR–Cas-mediated systems for site-specific RNA editing. Cell Rep. 33, 108350 (2020).
Colognori, D., Trinidad, M. & Doudna, J. A. Precise transcript targeting by CRISPR–Csm complexes. Nat. Biotechnol. 41, 1256–1264 (2023).
Abudayyeh, O. O. et al. RNA targeting with CRISPR–Cas13. Nature 550, 280–284 (2017).
Cox, D. B. T. et al. RNA editing with CRISPR–Cas13. Science 358, 1019–1027 (2017). The first study, to our knowledge, to achieve programmable RNA A-to-I base editing through a CRISPR-based RNA targeting system.
Stafforst, T. & Schneider, M. F. An RNA-deaminase conjugate selectively repairs point mutations. Angew. Chem. Int. Ed. Engl. 51, 11166–11169 (2012).
Vogel, P., Schneider, M. F., Wettengel, J. & Stafforst, T. Improving site-directed RNA editing in vitro and in cell culture by chemical modification of the guideRNA. Angew. Chem. Int. Ed. Engl. 53, 6267–6271 (2014).
Hanswillemenke, A., Kuzdere, T., Vogel, P., Jékely, G. & Stafforst, T. Site-directed RNA editing in vivo can be triggered by the light-driven assembly of an artificial riboprotein. J. Am. Chem. Soc. 137, 15875–15881 (2015).
Vogel, P. et al. Efficient and precise editing of endogenous transcripts with SNAP-tagged ADARs. Nat. Methods 15, 535–538 (2018).
Stroppel, A. S. et al. Harnessing self-labeling enzymes for selective and concurrent A-to-I and C-to-U RNA base editing. Nucleic Acids Res. 49, e95 (2021).
Schneider, M. F., Wettengel, J., Hoffmann, P. C. & Stafforst, T. Optimal guideRNAs for re-directing deaminase activity of hADAR1 and hADAR2 in trans. Nucleic Acids Res. 42, e87 (2014).
Montiel-González, M. F., Vallecillo-Viejo, I. C. & Rosenthal, J. J. An efficient system for selectively altering genetic information within mRNAs. Nucleic Acids Res. 44, e157 (2016).
Sinnamon, J. R. et al. Site-directed RNA repair of endogenous Mecp2 RNA in neurons. Proc. Natl Acad. Sci. USA 114, E9395–e9402 (2017).
Vallecillo-Viejo, I. C., Liscovitch-Brauer, N., Montiel-Gonzalez, M. F., Eisenberg, E. & Rosenthal, J. J. C. Abundant off-target edits from site-directed RNA editing can be reduced by nuclear localization of the editing enzyme. RNA Biol. 15, 104–114 (2018).
Montiel-Gonzalez, M. F., Vallecillo-Viejo, I., Yudowski, G. A. & Rosenthal, J. J. Correction of mutations within the cystic fibrosis transmembrane conductance regulator by site-directed RNA editing. Proc. Natl Acad. Sci. USA 110, 18285–18290 (2013).
Sinnamon, J. R. et al. In vivo repair of a protein underlying a neurological disorder by programmable RNA editing. Cell Rep. 32, 107878 (2020).
Katrekar, D. et al. In vivo RNA editing of point mutations via RNA-guided adenosine deaminases. Nat. Methods 16, 239–242 (2019).
Tohama, T., Sakari, M. & Tsukahara, T. Development of a single construct system for site-directed RNA editing using MS2–ADAR. Int. J. Mol. Sci. 21, 4943 (2020).
Azad, M. T. A., Bhakta, S. & Tsukahara, T. Site-directed RNA editing by adenosine deaminase acting on RNA for correction of the genetic code in gene therapy. Gene Ther. 24, 779–786 (2017).
Bhakta, S., Azad, M. T. A. & Tsukahara, T. Genetic code restoration by artificial RNA editing of Ochre stop codon with ADAR1 deaminase. Protein Eng. Des. Sel. 31, 471–478 (2018).
Rauch, S. et al. Programmable RNA-guided RNA effector proteins built from human parts. Cell 178, 122–134 (2019).
Han, W. et al. Programmable RNA base editing with a single gRNA-free enzyme. Nucleic Acids Res. 50, 9580–9595 (2022).
Royan, S. et al. A synthetic RNA editing factor edits its target site in chloroplasts and bacteria. Commun. Biol. 4, 545 (2021).
Ichinose, M. et al. U-to-C RNA editing by synthetic PPR-DYW proteins in bacteria and human culture cells. Commun. Biol. 5, 968 (2022).
Reautschnig, P. et al. CLUSTER guide RNAs enable precise and efficient RNA editing with endogenous ADAR enzymes in vivo. Nat. Biotechnol. 40, 759–768 (2022). The CLUSTER approach enhanced editing efficiency and reduced bystander editing.
Monian, P. et al. Endogenous ADAR-mediated RNA editing in non-human primates using stereopure chemically modified oligonucleotides. Nat. Biotechnol. 40, 1093–1102 (2022). The first study, to our knowledge, that applied A-to-I editor in non-human primates.
Qu, L. et al. Programmable RNA editing by recruiting endogenous ADAR using engineered RNAs. Nat. Biotechnol. 37, 1059–1069 (2019).
Merkle, T. et al. Precise RNA editing by recruiting endogenous ADARs with antisense oligonucleotides. Nat. Biotechnol. 37, 133–138 (2019).
Yi, Z. et al. Engineered circular ADAR-recruiting RNAs increase the efficiency and fidelity of RNA editing in vitro and in vivo. Nat. Biotechnol. 40, 946–955 (2022).
Katrekar, D. et al. Efficient in vitro and in vivo RNA editing via recruitment of endogenous ADARs using circular guide RNAs. Nat. Biotechnol. 40, 938–945 (2022). These two independent studies use circular guide RNAs to recruit endogenous ADAR.
Song, J. et al. CRISPR-free, programmable RNA pseudouridylation to suppress premature termination codons. Mol. Cell 83, 139–155 (2023).
Adachi, H. et al. Targeted pseudouridylation: an approach for suppressing nonsense mutations in disease genes. Mol. Cell 83, 637–651 (2023). Programmable U-to-Ψ base editors rely on guide snoRNA to revert PTC-induced translation termination in mammalian cells.
Zhao, X. & Yu, Y. T. Targeted pre-mRNA modification for gene silencing and regulation. Nat. Methods 5, 95–100 (2008).
Scheitl, C. P. M., Ghaem Maghami, M., Lenz, A. K. & Höbartner, C. Site-specific RNA methylation by a methyltransferase ribozyme. Nature 587, 663–667 (2020).
Booth, B. J. et al. RNA editing: expanding the potential of RNA therapeutics. Mol. Ther. 31, 1533–1549 (2023).
Abudayyeh, O. O. et al. A cytosine deaminase for programmable single-base RNA editing. Science 365, 382–386 (2019). The first study, to our knowledge, to achieve programmable RNA C-to-U base editing.
Huang, X. et al. Programmable C-to-U RNA editing using the human APOBEC3A deaminase. EMBO J. 39, e104741 (2020).
Gao, M. et al. Targeted manipulation of cellular RNA m6A methylation at the single-base level. ACS Chem. Biol. 17, 854–863 (2022).
Wilson, C., Chen, P. J., Miao, Z. & Liu, D. R. Programmable m6A modification of cellular RNAs with a Cas13-directed methyltransferase. Nat. Biotechnol. 38, 1431–1440 (2020).
Liu, X. M., Zhou, J., Mao, Y., Ji, Q. & Qian, S. B. Programmable RNA N6-methyladenosine editing by CRISPR–Cas9 conjugates. Nat. Chem. Biol. 15, 865–871 (2019).
Liu, Y. et al. REPAIRx, a specific yet highly efficient programmable A>I RNA base editor. EMBO J. 39, e104748 (2020).
Xu, C. et al. Programmable RNA editing with compact CRISPR–Cas13 systems from uncultivated microbes. Nat. Methods 18, 499–506 (2021).
Li, G. et al. Mini-dCas13X-mediated RNA editing restores dystrophin expression in a humanized mouse model of Duchenne muscular dystrophy. J. Clin. Invest. 133, e162809 (2023).
Xiao, Q. et al. Rescue of autosomal dominant hearing loss by in vivo delivery of mini dCas13X-derived RNA base editor. Sci. Transl. Med. 14, eabn0449 (2022).
Kannan, S. et al. Compact RNA editors with small Cas13 proteins. Nat. Biotechnol. 40, 194–197 (2022).
Latifi, N., Mack, A. M., Tellioglu, I., Di Giorgio, S. & Stafforst, T. Precise and efficient C-to-U RNA base editing with SNAP-CDAR-S. Nucleic Acids Res. 51, e84 (2023).
Bhakta, S., Sakari, M. & Tsukahara, T. RNA editing of BFP, a point mutant of GFP, using artificial APOBEC1 deaminase to restore the genetic code. Sci. Rep. 10, 17304 (2020).
Wettengel, J., Reautschnig, P., Geisler, S., Kahle, P. J. & Stafforst, T. Harnessing human ADAR2 for RNA repair—recoding a PINK1 mutation rescues mitophagy. Nucleic Acids Res. 45, 2797–2808 (2017).
Fukuda, M. et al. Construction of a guide-RNA for site-directed RNA mutagenesis utilising intracellular A-to-I RNA editing. Sci. Rep. 7, 41478 (2017).
Kaseniit, K. E. et al. Modular, programmable RNA sensing using ADAR editing in living cells. Nat. Biotechnol. 41, 482–487 (2023).
Jiang, K. et al. Programmable eukaryotic protein synthesis with RNA sensors by harnessing ADAR. Nat. Biotechnol. 41, 698–707 (2023).
Qian, Y. et al. Programmable RNA sensing for cell monitoring and manipulation. Nature 610, 713–721 (2022).
Wang, J., Zhao, Y.-T., Hu, L.-F. & Wang, Y. Monitoring promoter activity by RNA editing based reporter. Preprint at bioRxiv https://doi.org/10.1101/2022.03.30.486490 (2022).
Ge, J. & Yu, Y. T. RNA pseudouridylation: new insights into an old modification. Trends Biochem. Sci. 38, 210–218 (2013).
Karikó, K., Buckstein, M., Ni, H. & Weissman, D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 23, 165–175 (2005).
Karikó, K. et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 16, 1833–1840 (2008).
Dolgin, E. CureVac COVID vaccine let-down spotlights mRNA design challenges. Nature 594, 483 (2021).
Karijolich, J. & Yu, Y. T. Converting nonsense codons into sense codons by targeted pseudouridylation. Nature 474, 395–398 (2011).
Karijolich, J., Yi, C. & Yu, Y. T. Transcriptome-wide dynamics of RNA pseudouridylation. Nat. Rev. Mol. Cell Biol. 16, 581–585 (2015).
Song, J. & Yi, C. Reading chemical modifications in the transcriptome. J. Mol. Biol. 432, 1824–1839 (2019).
Kiss, T., Fayet-Lebaron, E. & Jády, B. E. Box H/ACA small ribonucleoproteins. Mol. Cell 37, 597–606 (2010).
Angrisani, A., Turano, M., Paparo, L., Di Mauro, C. & Furia, M. A new human dyskerin isoform with cytoplasmic localization. Biochim. Biophys. Acta 1810, 1361–1368 (2011).
Belli, V. et al. A dynamic link between H/ACA snoRNP components and cytoplasmic stress granules. Biochim. Biophys. Acta Mol. Cell. Res. 1866, 118529 (2019).
Dabrowski, M., Bukowy-Bieryllo, Z. & Zietkiewicz, E. Translational readthrough potential of natural termination codons in eucaryotes—the impact of RNA sequence. RNA Biol. 12, 950–958 (2015).
Welch, E. M. et al. PTC124 targets genetic disorders caused by nonsense mutations. Nature 447, 87–91 (2007).
Mort, M., Ivanov, D., Cooper, D. N. & Chuzhanova, N. A. A meta-analysis of nonsense mutations causing human genetic disease. Hum. Mutat. 29, 1037–1047 (2008).
Adachi, H. & Yu, Y. T. Pseudouridine-mediated stop codon readthrough in S. cerevisiae is sequence context-independent. RNA 26, 1247–1256 (2020).
Dabrowski, M., Bukowy-Bieryllo, Z. & Zietkiewicz, E. Advances in therapeutic use of a drug-stimulated translational readthrough of premature termination codons. Mol. Med. 24, 25 (2018).
Roy, B. et al. Ataluren stimulates ribosomal selection of near-cognate tRNAs to promote nonsense suppression. Proc. Natl Acad. Sci. USA 113, 12508–12513 (2016).
Charlesworth, C. T. et al. Identification of preexisting adaptive immunity to Cas9 proteins in humans. Nat. Med. 25, 249–254 (2019).
Tang, X. E., Tan, S. X., Hoon, S. & Yeo, G. W. Pre-existing adaptive immunity to the RNA-editing enzyme Cas13d in humans. Nat. Med. 28, 1372–1376 (2022).
Teoh, P. J. et al. Aberrant hyperediting of the myeloma transcriptome by ADAR1 confers oncogenicity and is a marker of poor prognosis. Blood 132, 1304–1317 (2018).
Dai, Q. et al. Quantitative sequencing using BID-seq uncovers abundant pseudouridines in mammalian mRNA at base resolution. Nat. Biotechnol. 41, 344–354 (2023).
Small, I. D., Schallenberg-Rüdinger, M., Takenaka, M., Mireau, H. & Ostersetzer-Biran, O. Plant organellar RNA editing: what 30 years of research has revealed. Plant J. 101, 1040–1056 (2020).
Yin, Q. F. et al. Long noncoding RNAs with snoRNA ends. Mol. Cell 48, 219–230 (2012).
Klimek-Tomczak, K. et al. Editing of hnRNP K protein mRNA in colorectal adenocarcinoma and surrounding mucosa. Br. J. Cancer 94, 586–592 (2006).
Niavarani, A. et al. APOBEC3A is implicated in a novel class of G-to-A mRNA editing in WT1 transcripts. PLoS ONE 10, e0120089 (2015).
Li, X. et al. Base-resolution mapping reveals distinct m1A methylome in nuclear- and mitochondrial-encoded transcripts. Mol. Cell 68, 993–1005 (2017).
Guo, J. & Niu, W. Genetic code expansion through quadruplet codon decoding. J. Mol. Biol. 434, 167346 (2022).
Roundtree, I. A., Evans, M. E., Pan, T. & He, C. Dynamic RNA modifications in gene expression regulation. Cell 169, 1187–1200 (2017).
Anzalone, A. V. et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576, 149–157 (2019).
Rauch, S., Jones, K. A. & Dickinson, B. C. Small molecule-inducible RNA-targeting systems for temporal control of RNA regulation. ACS Cent. Sci. 6, 1987–1996 (2020).
Acknowledgements
This work was supported by the National Key Research and Development Program (grant no. 2019YFA0802201 to C.Y.), the National Natural Science Foundation of China (grant nos. 92153303 and 21825701 to C.Y.), the Ministry of Agriculture and Rural Affairs of China (grant no. NK2022010102 to C.Y.) and funding from the Beijing Municipal Science & Technology Commission (grant no. Z231100002723005 to C.Y.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
C.Y. is a founder of Modit therapeutics. J.S. and Y.Z. declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Bryan Dickinson and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, J., Zhuang, Y. & Yi, C. Programmable RNA base editing via targeted modifications. Nat Chem Biol 20, 277–290 (2024). https://doi.org/10.1038/s41589-023-01531-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-023-01531-y