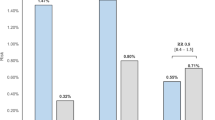
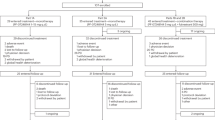
Abstract
The discovery of the benefits of castration for prostate cancer treatment in 1941 led to androgen deprivation therapy, which remains a mainstay of the treatment of men with advanced prostate cancer. However, as early as this original publication, the inevitable development of castration-resistant prostate cancer was recognized. Resistance first manifests as a sustained rise in the androgen-responsive gene, PSA, consistent with reactivation of the androgen receptor axis. Evaluation of clinical specimens demonstrates that castration-resistant prostate cancer cells remain addicted to androgen signalling and adapt to chronic low-testosterone states. Paradoxically, results of several studies have suggested that treatment with supraphysiological levels of testosterone can retard prostate cancer growth. Insights from these studies have been used to investigate administration of supraphysiological testosterone to patients with prostate cancer for clinical benefits, a strategy that is termed bipolar androgen therapy (BAT). BAT involves rapid cycling from supraphysiological back to near-castration testosterone levels over a 4-week cycle. Understanding how BAT works at the molecular and cellular levels might help to rationalize combining BAT with other agents to achieve increased efficacy and tumour responses.
Key points
-
Androgens can drive prostate cancer growth providing the rationale for using deprivation of androgens as a first line of treatment for prostate cancer. Unfortunately, prostate cancer cells adapt to low androgen levels and eventually progress to a castration-resistant state.
-
Results of several studies have indicated a paradoxical decrease in tumour growth in prostate cancer models upon treatment with supraphysiological levels of testosterone. Evidence indicates several complementary mechanisms, including cell death and cytostasis, which might be responsible for paradoxical growth inhibition by supraphysiological testosterone.
-
Adaptive reliance on androgen signalling by castration-resistant prostate cancer cells becomes a therapeutic liability that can be exploited clinically through the administration of supraphysiological testosterone, an approach termed ‘bipolar androgen therapy’ (BAT). The term bipolar is used to emphasize that, with this strategy, rapid cycling occurs between two extremes: from supraphysiological back to near-castration testosterone levels over a 4-week cycle.
-
Understanding how BAT works at the molecular and cellular levels might help to develop biomarkers for patient stratification and to rationally combine BAT with other agents to achieve increased efficacy.
This is a preview of subscription content, access via your institution
Access options



Similar content being viewed by others
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Butenandt, A. Über die chemische Untersuchung des SexualHormons. Angew. Chem. 44, 905–908 (1931).
Butenandt, A. & Tscherning, K. Androsterone, a crystalline male sex hormone. I. Isolation and purification from male urine. Z. Physiol. Chem. 229, 167 (1934).
David K, D. E., Freud, J. & Laqueur, E. Über krystallinisches männliches Hormon aus Hoden (Testosteron), wirksamer als aus Harn oder aus Cholesterin bereitetes Androsteron. Hoppe Seylers Z. Physiol. Chem. 233, 281–283 (1935).
Pearlman, W. H. & Crepy, O. Steroid-protein interaction with particular reference to testosterone binding by human serum. J. Biol. Chem. 242, 182–189 (1967).
Rosner, W. & Deakins, S. M. Testosterone-binding globulins in human plasma: studies on sex distribution and specificity. J. Clin. Invest. 47, 2109–2116 (1968).
Pearlman, W. H. & Pearlman, M. R. The metabolism in vivo of Δ4-androstene-3, 17-dione-7-H3; its localization in the ventral prostate and other tissues of the rat. J. Biol. Chem. 236, 1321–1327 (1961).
Fang, S., Anderson, K. M. & Liao, S. Receptor proteins for androgens. On the role of specific proteins in selective retention of 17-β-hydroxy-5-α-androstan-3-one by rat ventral prostate in vivo and in vitro. J. Biol. Chem. 244, 6584–6595 (1969).
Bruchovsky, N. & Wilson, J. D. The conversion of testosterone to 5-α-androstan-17-β-ol-3-one by rat prostate in vivo and in vitro. J. Biol. Chem. 243, 2012–2021 (1968).
Imperato-McGinley, J., Guerrero, L., Gautier, T. & Peterson, R. E. Steroid 5α-reductase deficiency in man: an inherited form of male pseudohermaphroditism. Science 186, 1213–1215 (1974).
Siiteri, P. K. & Wilson, J. D. Testosterone formation and metabolism during male sexual differentiation in the human embryo. J. Clin. Endocrinol. Metab. 38, 113–125 (1974).
Anderson, K. M. & Liao, S. Selective retention of dihydrotestosterone by prostatic nuclei. Nature 219, 277–279 (1968).
Lubahn, D. B. et al. Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science 240, 327–330 (1988).
Chang, C. S., Kokontis, J. & Liao, S. T. Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science 240, 324–326 (1988).
Mangelsdorf, D. J. et al. The nuclear receptor superfamily: the second decade. Cell 83, 835–839 (1995).
Velasco, A. M. et al. Identification and validation of novel androgen-regulated genes in prostate cancer. Endocrinology 145, 3913–3924 (2004).
Sahu, B. et al. FoxA1 specifies unique androgen and glucocorticoid receptor binding events in prostate cancer cells. Cancer Res. 73, 1570–1580 (2013).
Gao, S. et al. Chromatin binding of FOXA1 is promoted by LSD1-mediated demethylation in prostate cancer. Nat. Genet. 52, 1011–1017 (2020).
Sahu, B. et al. Dual role of FoxA1 in androgen receptor binding to chromatin, androgen signalling and prostate cancer. EMBO J. 30, 3962–3976 (2011).
Jia, L. et al. Genomic androgen receptor-occupied regions with different functions, defined by histone acetylation, coregulators and transcriptional capacity. PLoS One 3, e3645 (2008).
Visakorpi, T. et al. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat. Genet. 9, 401–406 (1995).
Li, Y. et al. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res. 73, 483–489 (2013).
Huggins, C. & Hodges, C. V. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1, 293–297 (1941).
Fu, A. Z. et al. Mortality and androgen deprivation therapy as salvage treatment for biochemical recurrence after primary therapy for clinically localized prostate cancer. J. Urol. 197, 1448–1454 (2017).
Sharifi, N., Gulley, J. L. & Dahut, W. L. Androgen deprivation therapy for prostate cancer. JAMA 294, 238–244 (2005).
Tangen, C. M. et al. Ten-year survival in patients with metastatic prostate cancer. Clin. Prostate Cancer 2, 41–45 (2003).
Pienta, K. J. & Bradley, D. Mechanisms underlying the development of androgen-independent prostate cancer. Clin. Cancer Res. 12, 1665–1671 (2006).
Denmeade, S. R. & Isaacs, J. T. Bipolar androgen therapy: the rationale for rapid cycling of supraphysiologic androgen/ablation in men with castration resistant prostate cancer. Prostate 70, 1600–1607 (2010).
McNeal, J. E. Regional morphology and pathology of the prostate. Am. J. Clin. Pathol. 49, 347–357 (1968).
McNeal, J. E. Normal histology of the prostate. Am. J. Surg. Pathol. 12, 619–633 (1988).
Cunha, G. R. & Chung, L. W. Stromal-epithelial interactions — I. Induction of prostatic phenotype in urothelium of testicular feminized (Tfm/y) mice. J. Steroid Biochem. 14, 1317–1324 (1981).
Cunha, G. R. et al. Normal and abnormal development of the male urogenital tract. Role of androgens, mesenchymal-epithelial interactions, and growth factors. J. Androl. 13, 465–475 (1992).
Isaacs, J. T. & Coffey, D. S. Etiology and disease process of benign prostatic hyperplasia. Prostate Suppl. 2, 33–50 (1989).
English, H. F., Santen, R. J. & Isaacs, J. T. Response of glandular versus basal rat ventral prostatic epithelial cells to androgen withdrawal and replacement. Prostate 11, 229–242 (1987).
Collins, A. T., Habib, F. K., Maitland, N. J. & Neal, D. E. Identification and isolation of human prostate epithelial stem cells based on α2β1-integrin expression. J. Cell Sci. 114, 3865–3872 (2001).
Bonkhoff, H. & Remberger, K. Widespread distribution of nuclear androgen receptors in the basal cell layer of the normal and hyperplastic human prostate. Virchows Arch. A Pathol. Anat. Histopathol. 422, 35–38 (1993).
Bonkhoff, H., Stein, U. & Remberger, K. The proliferative function of basal cells in the normal and hyperplastic human prostate. Prostate 24, 114–118 (1994).
Germann, M. et al. Stem-like cells with luminal progenitor phenotype survive castration in human prostate cancer. Stem Cell 30, 1076–1086 (2012).
Wang, X. et al. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature 461, 495–500 (2009).
Choi, N., Zhang, B., Zhang, L., Ittmann, M. & Xin, L. Adult murine prostate basal and luminal cells are self-sustained lineages that can both serve as targets for prostate cancer initiation. Cancer Cell 21, 253–265 (2012).
Ousset, M. et al. Multipotent and unipotent progenitors contribute to prostate postnatal development. Nat. Cell Biol. 14, 1131–1138 (2012).
Wang, Z. A. et al. Lineage analysis of basal epithelial cells reveals their unexpected plasticity and supports a cell-of-origin model for prostate cancer heterogeneity. Nat. Cell Biol. 15, 274–283 (2013).
Wu, X. et al. Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech. Dev. 101, 61–69 (2001).
Xie, Q. et al. Dissecting cell-type-specific roles of androgen receptor in prostate homeostasis and regeneration through lineage tracing. Nat. Commun. 8, 14284 (2017).
Karthaus, W. R. et al. Regenerative potential of prostate luminal cells revealed by single-cell analysis. Science 368, 497–505 (2020).
Dai, C., Heemers, H. & Sharifi, N. Androgen signaling in prostate cancer. Cold Spring Harb. Perspect. Med. https://doi.org/10.1101/cshperspect.a030452 (2017).
Kumar, A. et al. Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer. Nat. Med. 22, 369–378 (2016).
Ware, K. E., Garcia-Blanco, M. A., Armstrong, A. J. & Dehm, S. M. Biologic and clinical significance of androgen receptor variants in castration resistant prostate cancer. Endocr. Relat. Cancer 21, T87–T103 (2014).
Cancer Genome Atlas Research Network. The molecular taxonomy of primary prostate cancer. Cell 163, 1011–1025 (2015).
Chen, E. J. et al. Abiraterone treatment in castration-resistant prostate cancer selects for progesterone responsive mutant androgen receptors. Clin. Cancer Res. 21, 1273–1280 (2015).
Korpal, M. et al. An F876L mutation in androgen receptor confers genetic and phenotypic resistance to MDV3100 (enzalutamide). Cancer Discov. 3, 1030–1043 (2013).
Gottlieb, B., Beitel, L. K., Wu, J. H. & Trifiro, M. The androgen receptor gene mutations database (ARDB): 2004 update. Hum. Mutat. 23, 527–533 (2004).
Robinson, J. L. et al. Elevated levels of FOXA1 facilitate androgen receptor chromatin binding resulting in a CRPC-like phenotype. Oncogene 33, 5666–5674 (2014).
Wang, Q. et al. A hierarchical network of transcription factors governs androgen receptor-dependent prostate cancer growth. Mol. Cell 27, 380–392 (2007).
Pomerantz, M. M. et al. The androgen receptor cistrome is extensively reprogrammed in human prostate tumorigenesis. Nat. Genet. 47, 1346–1351 (2015).
Stelloo, S., Bergman, A. M. & Zwart, W. Androgen receptor enhancer usage and the chromatin regulatory landscape in human prostate cancers. Endocr. Relat. Cancer 26, R267–R285 (2019).
Westaby, D. et al. A new old target: androgen receptor signaling and advanced prostate cancer. Annu. Rev. Pharmacol. Toxicol. 62, 131–153 (2022).
Uo, T., Sprenger, C. C. & Plymate, S. R. Androgen receptor signaling and metabolic and cellular plasticity during progression to castration resistant prostate cancer. Front. Oncol. 10, 580617 (2020).
Culig, Z. & Santer, F. R. Androgen receptor signaling in prostate cancer. Cancer Metastasis Rev. 33, 413–427 (2014).
Deng, Q. et al. Non-genomic action of androgens is mediated by rapid phosphorylation and regulation of androgen receptor trafficking. Cell. Physiol. Biochem. 43, 223–236 (2017).
Leung, J. K. & Sadar, M. D. Non-genomic actions of the androgen receptor in prostate cancer. Front. Endocrinol. 8, 2 (2017).
Zarif, J. C. & Miranti, C. K. The importance of non-nuclear AR signaling in prostate cancer progression and therapeutic resistance. Cell Signal. 28, 348–356 (2016).
Harris, W. P., Mostaghel, E. A., Nelson, P. S. & Montgomery, B. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat. Clin. Pract. Urol. 6, 76–85 (2009).
Huggins, C. & Scott, W. W. Bilateral adrenalectomy in prostatic cancer: clinical features and urinary excretion of 17-ketosteroids and estrogen. Ann. Surg. 122, 1031–1041 (1945).
de Bono, J. S. et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 364, 1995–2005 (2011).
Ryan, C. J., Smith, M. R. & Bono, J. S. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 368, 138–148 (2013).
Fizazi, K., Tran, N. & Fein, L. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N. Engl. J. Med. 377, 352–360 (2017).
Liao, S., Howell, D. K. & Chang, T. M. Action of a nonsteroidal antiandrogen, flutamide, on the receptor binding and nuclear retention of 5 α-dihydrotestosterone in rat ventral prostate. Endocrinology 94, 1205–1209 (1974).
Tran, C. et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 324, 787–790 (2009).
Beer, T. M. et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 371, 424–433 (2014).
Chi, K. N. et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N. Engl. J. Med. 381, 13–24 (2019).
Fizazi, K. et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N. Engl. J. Med. 380, 1235–1246 (2019).
Hussain, M. et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N. Engl. J. Med. 378, 2465–2474 (2018).
Scher, H. I. et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 367, 1187–1197 (2012).
Maron, S. B. et al. Pembrolizumab with trastuzumab and chemotherapy (PTC) in HER2-positive metastatic esophagogastric cancer (mEG): plasma and tumor-based biomarker analysis. J. Clin. Oncol. 38 (Suppl. 15), 4559 (2020).
ClinicalTrials.gov. US National Library of Medicine. https://ClinicalTrials.gov/show/NCT03888612 (2021).
Linja, M. J., Savinainen, K. J. & Saramäki, O. R. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 61, 3550–3555 (2001).
Azad, A. A., Volik, S. V. & Wyatt, A. W. Androgen receptor gene aberrations in circulating cell-free DNA: biomarkers of therapeutic resistance in castration-resistant prostate cancer. Clin. Cancer Res. 21, 2315–2324 (2015).
Isaacs, J. T. & Isaacs, W. B. Androgen receptor outwits prostate cancer drugs. Nat. Med 10, 26–27 (2004).
Antonarakis, E. S., Lu, C. & Wang, H. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 371, 1028–1038 (2014).
Scher, H. I. & Sawyers, C. L. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J. Clin. Oncol. 23, 8253–8261 (2005).
Huggins, C. & Yang, N. C. Induction and extinction of mammary cancer. A striking effect of hydrocarbons permits analysis of mechanisms of causes and cure of breast cancer. Science 137, 257–262 (1962).
Horoszewicz, J. S. et al. LNCaP model of human prostatic carcinoma. Cancer Res. 43, 1809–1818 (1983).
Berns, E. M., de Boer, W. & Mulder, E. Androgen-dependent growth regulation of and release of specific protein(s) by the androgen receptor containing human prostate tumor cell line LNCaP. Prostate 9, 247–259 (1986).
Dai, J. L., Maiorino, C. A., Gkonos, P. J. & Burnstein, K. L. Androgenic up-regulation of androgen receptor cDNA expression in androgen-independent prostate cancer cells. Steroids 61, 531–539 (1996).
Kokontis, J., Takakura, K., Hay, N. & Liao, S. Increased androgen receptor activity and altered c-myc expression in prostate cancer cells after long-term androgen deprivation. Cancer Res. 54, 1566–1573 (1994).
Heisler, L. E. et al. Androgen-dependent cell cycle arrest and apoptotic death in PC-3 prostatic cell cultures expressing a full-length human androgen receptor. Mol. Cell. Endocrinol. 126, 59–73 (1997).
Kokontis, J. M. et al. Androgen suppresses the proliferation of androgen receptor-positive castration-resistant prostate cancer cells via inhibition of Cdk2, CyclinA, and Skp2. PLoS One 9, e109170 (2014).
Ling, M. T., Chan, K. W. & Choo, C. K. Androgen induces differentiation of a human papillomavirus 16 E6/E7 immortalized prostate epithelial cell line. J. Endocrinol. 170, 287–296 (2001).
Berthon, P. et al. Androgens are not a direct requirement for the proliferation of human prostatic epithelium in vitro. Int. J. Cancer 73, 910–916 (1997).
Antony, L., van der Schoor, F., Dalrymple, S. L. & Isaacs, J. T. Androgen receptor (AR) suppresses normal human prostate epithelial cell proliferation via AR/β-catenin/TCF-4 complex inhibition of c-MYC transcription. Prostate 74, 1118–1131 (2014).
D’Antonio, J. M., Vander Griend, D. J. & Isaacs, J. T. DNA licensing as a novel androgen receptor mediated therapeutic target for prostate cancer. Endocr. Relat. Cancer 16, 325–332 (2009).
Vander Griend, D. J., Litvinov, I. V. & Isaacs, J. T. Stabilizing androgen receptor in mitosis inhibits prostate cancer proliferation. Cell Cycle 6, 647–651 (2007).
Litvinov, I. V. et al. Androgen receptor as a licensing factor for DNA replication in androgen-sensitive prostate cancer cells. Proc. Natl Acad. Sci. USA 103, 15085–15090 (2006).
Fragkos, M., Ganier, O., Coulombe, P. & Mechali, M. DNA replication origin activation in space and time. Nat. Rev. Mol. Cell Biol. 16, 360–374 (2015).
Nishitani, H., Taraviras, S., Lygerou, Z. & Nishimoto, T. The human licensing factor for DNA replication Cdt1 accumulates in G1 and is destabilized after initiation of S-phase. J. Biol. Chem. 276, 44905–44911 (2001).
Nishitani, H. & Lygerou, Z. Control of DNA replication licensing in a cell cycle. Genes Cell 7, 523–534 (2002).
Shi, Y. K. et al. MCM7 interacts with androgen receptor. Am. J. Pathol. 173, 1758–1767 (2008).
Wolf, D. A., Herzinger, T., Hermeking, H., Blaschke, D. & Horz, W. Transcriptional and posttranscriptional regulation of human androgen receptor expression by androgen. Mol. Endocrinol. 7, 924–936 (1993).
Henttu, P. & Vihko, P. Growth factor regulation of gene expression in the human prostatic carcinoma cell line LNCaP. Cancer Res. 53, 1051–1058 (1993).
Cai, C. et al. Androgen receptor gene expression in prostate cancer is directly suppressed by the androgen receptor through recruitment of lysine-specific demethylase 1. Cancer Cell 20, 457–471 (2011).
Rudolph, T., Beuch, S. & Reuter, G. Lysine-specific histone demethylase LSD1 and the dynamic control of chromatin. Biol. Chem. 394, 1019–1028 (2013).
Cerella, C., Grandjenette, C., Dicato, M. & Diederich, M. Roles of apoptosis and cellular senescence in cancer and aging. Curr. Drug. Targets 17, 405–415 (2016).
Wang, X., Deng, H., Basu, I. & Zhu, L. Induction of androgen receptor-dependent apoptosis in prostate cancer cells by the retinoblastoma protein. Cancer Res. 64, 1377–1385 (2004).
Lin, Y. et al. Androgen and its receptor promote Bax-mediated apoptosis. Mol. Cell Biol. 26, 1908–1916 (2006).
Joly-Pharaboz, M. O. et al. Inhibition of growth and induction of apoptosis by androgens of a variant of LNCaP cell line. J. Steroid Biochem. Mol. Biol. 73, 237–249 (2000).
Roediger, J. et al. Supraphysiological androgen levels induce cellular senescence in human prostate cancer cells through the Src-Akt pathway. Mol. Cancer 13, 214 (2014).
Mirochnik, Y. et al. Androgen receptor drives cellular senescence. PLoS One 7, e31052 (2012).
Han, W. et al. Exploiting the tumor-suppressive activity of the androgen receptor by CDK4/6 inhibition in castration-resistant prostate cancer. Mol. Ther. https://doi.org/10.1016/j.ymthe.2022.01.039 (2022).
Demidenko, Z. N. et al. Rapamycin decelerates cellular senescence. Cell Cycle 8, 1888–1895 (2009).
Herranz, N. et al. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat. Cell Biol. 17, 1205–1217 (2015).
Bui, A. T. et al. Transient exposure to androgens induces a remarkable self-sustained quiescent state in dispersed prostate cancer cells. Cell Cycle 16, 879–893 (2017).
Ju, B. G. et al. A topoisomerase IIβ-mediated dsDNA break required for regulated transcription. Science 312, 1798–1802 (2006).
Lin, C. et al. Nuclear receptor-induced chromosomal proximity and DNA breaks underlie specific translocations in cancer. Cell 139, 1069–1083 (2009).
Haffner, M. C. et al. Androgen-induced TOP2B-mediated double-strand breaks and prostate cancer gene rearrangements. Nat. Genet. 42, 668–675 (2010).
Tomlins, S. A. et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310, 644–648 (2005).
Kim, N. & Jinks-Robertson, S. Transcription as a source of genome instability. Nat. Rev. Genet. 13, 204–214 (2012).
Cristini, A., Geraud, M. & Sordet, O. Transcription-associated DNA breaks and cancer: a matter of DNA topology. Int. Rev. Cell Mol. Biol. 364, 195–240 (2021).
Chatterjee, P. et al. Supraphysiological androgens suppress prostate cancer growth through androgen receptor-mediated DNA damage. J. Clin. Invest. 129, 4245–4260 (2019).
Lam, H. M. et al. Durable response of enzalutamide-resistant prostate cancer to supraphysiological testosterone is associated with a multifaceted growth suppression and impaired DNA damage response transcriptomic program in patient-derived xenografts. Eur. Urol. 77, 144–155 (2020).
Markowski, M. C. et al. Molecular and clinical characterization of patients with metastatic castration resistant prostate cancer achieving deep responses to bipolar androgen therapy. Clin. Genitourin. Cancer https://doi.org/10.1016/j.clgc.2021.08.001 (2021).
Markowski, M. C. et al. Extreme responses to immune checkpoint blockade following bipolar androgen therapy and enzalutamide in patients with metastatic castration resistant prostate cancer. Prostate 80, 407–411 (2020).
Teply, B. A., Kachhap, S., Eisenberger, M. A. & Denmeade, S. R. Extreme response to high-dose testosterone in BRCA2- and ATM-mutated prostate cancer. Eur. Urol. 71, 499 (2017).
Kumar, R. et al. Supraphysiologic testosterone induces ferroptosis and activates immune pathways through nucleophagy in prostate cancer. Cancer Res. 81, 5948–5962 (2021).
Torres-Estay, V. et al. Androgen receptor in human endothelial cells. J. Endocrinol. 224, R131–R137 (2015).
Mantalaris, A. et al. Localization of androgen receptor expression in human bone marrow. J. Pathol. 193, 361–366 (2001).
Blanquart, E., Laffont, S. & Guéry, J.-C. Sex hormone regulation of innate lymphoid cells. Biomed. J. 44, 144–156 (2021).
Guan, X. et al. Androgen receptor activity in T cells limits checkpoint blockade efficacy. Nature https://doi.org/10.1038/s41586-022-04522-6 (2022).
Lai, J.-J. et al. Monocyte/macrophage androgen receptor suppresses cutaneous wound healing in mice by enhancing local TNF-α expression. J. Clin. Invest. 119, 3739–3751 (2009).
Tagnon, H. J., Schulman, P., Whitmore, W. F. & Leone, L. A. Prostatic fibrinolysin: study of a case illustrating role in hemorrhagic diathesis of cancer of the prostate. Am. J. Med. 15, 875–884 (1953).
Bonner, C. D., Fishman, W. H. & Homburger, F. Serum prostatic acid phosphatase and cancer of the prostate. N. Engl. J. Med. 255, 925–933 (1956).
Fowler Jr, J. E. & Whitmore Jr, W. F. The response of metastatic adenocarcinoma of the prostate to exogenous testosterone. J. Urol. 126, 372–375 (1981).
Manni, A., Bartholomew, M. & Caplan, R. Androgen priming and chemotherapy in advanced prostate cancer: evaluation of determinants of clinical outcome. J. Clin. Oncol. 6, 1456–1466 (1988).
Suarez, A. J., Lamm, D. L. & Radwin, H. M. Androgen priming and cytotoxic chemotherapy in advanced prostatic cancer. Cancer Chemother. Pharmacol. 8, 261–265 (1982).
Donati, R. M., Ellis, H. & Gallagher, N. I. Testosterone potentiated 32P therapy in prostatic carcinoma. Cancer 19, 1088–1090 (1966).
Prout Jr, G. R. & Brewer, W. R. Response of men with advanced prostatic carcinoma to exogenous administration of testosterone. Cancer 20, 1871–1878 (1967).
Khera, M. et al. Testosterone replacement therapy following radical prostatectomy. J. Sex. Med. 6, 1165–1170 (2009).
Pastuszak, A. W. et al. Testosterone replacement therapy in patients with prostate cancer after radical prostatectomy. J. Urol. 190, 639–644 (2013).
Pastuszak, A. W. et al. Testosterone replacement therapy in the setting of prostate cancer treated with radiation. Int. J. Impot. Res. 25, 24–28 (2013).
Ahlering, T. E. et al. Testosterone replacement therapy reduces biochemical recurrence after radical prostatectomy. BJU Int. 126, 91–96 (2020).
Morgentaler, A. et al. Testosterone therapy in men with untreated prostate cancer. J. Urol. 185, 1256–1260 (2011).
Cui, Y., Zong, H., Yan, H. & Zhang, Y. The effect of testosterone replacement therapy on prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 17, 132–143 (2014).
Morris, M. J., Huang, D. & Kelly, W. K. Phase 1 trial of high-dose exogenous testosterone in patients with castration-resistant metastatic prostate cancer. Eur. Urol. 56, 237–244 (2009).
Szmulewitz, R., Mohile, S. & Posadas, E. A randomized phase 1 study of testosterone replacement for patients with low-risk castration-resistant prostate cancer. Eur. Urol. 56, 97–103 (2009).
Schweizer, M. T. et al. Effect of bipolar androgen therapy for asymptomatic men with castration-resistant prostate cancer: results from a pilot clinical study. Sci. Transl. Med. 7, 269ra2 (2015).
Umekita, Y., Hiipakka, R. A., Kokontis, J. M. & Liao, S. Human prostate tumor growth in athymic mice: inhibition by androgens and stimulation by finasteride. Proc. Natl Acad. Sci. USA 93, 11802–11807 (1996).
Schweizer, M. T. et al. Bipolar androgen therapy for men with androgen ablation naive prostate cancer: results from the phase II BATMAN study. Prostate 76, 1218–1226 (2016).
Markowski, M. C. et al. A multicohort open-label phase II trial of bipolar androgen therapy in men with metastatic castration-resistant prostate cancer (RESTORE): a comparison of post-abiraterone versus post-enzalutamide cohorts. Eur. Urol. 79, 692–699 (2021).
Sena, L. A. et al. Bipolar androgen therapy sensitizes castration-resistant prostate cancer to subsequent androgen receptor ablative therapy. Eur. J. Cancer 144, 302–309 (2021).
Teply, B. A. et al. Bipolar androgen therapy in men with metastatic castration-resistant prostate cancer after progression on enzalutamide: an open-label, phase 2, multicohort study. Lancet Oncol. 19, 76–86 (2018).
Denmeade, S. R. et al. TRANSFORMER: a randomized phase II study comparing bipolar androgen therapy versus enzalutamide in asymptomatic men with castration-resistant metastatic prostate cancer. J. Clin. Oncol. 39, 1371–1382 (2021).
Markowski, M. C. et al. COMBAT-CRPC: concurrent administration of bipolar androgen therapy (BAT) and nivolumab in men with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. https://doi.org/10.1200/JCO.2021.39.15_suppl.5014 (2021).
Schweizer, M. et al. 592P Bipolar androgen therapy (BAT) plus olaparib in men with metastatic castration-resistant prostate cancer (mCRPC). Ann. Oncol. 32, S639–S640 (2021).
Manni, A. et al. Androgen depletion and repletion as a means of potentiating the effect of cytotoxic chemotherapy in advanced prostate cancer. J. Steroid Biochem. 27, 551–556 (1987).
Johnson, D. & Haynie, T. Phosphorus-32 for intractable pain in carcinoma of prostate: analysis of androgen priming, parathormone rebound, and combination therapy. Urology 9, 137–139 (1977).
Schwartz, L. H. et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur. J. Cancer 62, 132–137 (2016).
Scher, H. I. et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J. Clin. Oncol. 34, 1402–1418 (2016).
Sena, L. A. et al. Prostate cancer androgen receptor activity dictates efficacy of bipolar androgen therapy through MYC. J. Clin. Invest. https://doi.org/10.1172/JCI162396 (2022).
ClinicalTrials.gov. US National Library of Medicine. https://ClinicalTrials.gov/show/NCT02090114 (2022).
ClinicalTrials.gov. US National Library of Medicine. https://ClinicalTrials.gov/show/NCT03522064 (2021).
Abida, W., Cyrta, J. & Heller, G. Genomic correlates of clinical outcome in advanced prostate cancer. Proc. Natl Acad. Sci. USA 116, 11428–11436 (2019).
ClinicalTrials.gov. US National Library of Medicine. https://ClinicalTrials.gov/show/NCT04363164 (2022).
Sena, L. A., Denmeade, S. R. & Antonarakis, E. S. Targeting the spectrum of immune checkpoints in prostate cancer. Expert. Rev. Clin. Pharmacol. 14, 1253–1266 (2021).
Hussain, M., Mateo, J. & Fizazi, K. Survival with olaparib in metastatic castration-resistant prostate cancer. N. Engl. J. Med. 383, 2345–2357 (2020).
Nyquist, M. D. et al. Selective androgen receptor modulators activate the canonical prostate cancer androgen receptor program and repress cancer growth. J. Clin. Invest. https://doi.org/10.1172/JCI146777 (2021).
D’Andrea, A. D. Mechanisms of PARP inhibitor sensitivity and resistance. DNA Repair. 71, 172–176 (2018).
Bouman, A., Heineman, M. J. & Faas, M. M. Sex hormones and the immune response in humans. Hum. Reprod. Update 11, 411–423 (2005).
Isaacs, J. T. Resolving the Coffey Paradox: what does the androgen receptor do in normal vs. malignant prostate epithelial cells? Am. J. Clin. Exp. Urol. 6, 55–61 (2018).
Notelovitz, M. Androgen effects on bone and muscle. Fertil. Steril. 77 (Suppl. 4), S34–S41 (2002).
Lu, S., Tsai, S. Y. & Tsai, M.-J. Regulation of androgen-dependent prostatic cancer cell growth: androgen regulation of CDK2, CDK4, and CKI p16 genes. Cancer Res. 57, 4511–4516 (1997).
Berger, M. F. et al. The genomic complexity of primary human prostate cancer. Nature 470, 214–220 (2011).
Chuang, K.-H. et al. Neutropenia with impaired host defense against microbial infection in mice lacking androgen receptor. J. Exp. Med. 206, 1181–1199 (2009).
Tomlins, S. A. et al. Role of the TMPRSS2-ERG gene fusion in prostate cancer. Neoplasia 10, 177–IN179 (2008).
Tomlins, S. A. et al. ETS gene fusions in prostate cancer: from discovery to daily clinical practice. Eur. Urol. 56, 275–286 (2009).
Heemers, H. V. & Tindall, D. J. Unraveling the complexities of androgen receptor signaling in prostate cancer cells. Cancer Cell 15, 245–247 (2009).
Sharma, N. L. et al. The androgen receptor induces a distinct transcriptional program in castration-resistant prostate cancer in man. Cancer Cell 23, 35–47 (2013).
Wang, Q. et al. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell 138, 245–256 (2009).
Chuu, C. P. et al. Androgen suppresses proliferation of castration‐resistant LNCaP 104‐R2 prostate cancer cells through androgen receptor, Skp2, and c‐Myc. Cancer Sci. 102, 2022–2028 (2011).
Kokontis, J. M., Hay, N. & Liao, S. Progression of LNCaP prostate tumor cells during androgen deprivation: hormone-independent growth, repression of proliferation by androgen, and role for p27Kip1 in androgen-induced cell cycle arrest. Mol. Endocrinol. 12, 941–953 (1998).
Cornforth, A., Davis, J., Khanifar, E., Nastiuk, K. & Krolewski, J. FOXO3a mediates the androgen-dependent regulation of FLIP and contributes to TRAIL-induced apoptosis of LNCaP cells. Oncogene 27, 4422–4433 (2008).
Wang, Y. et al. Regulation of androgen receptor transcriptional activity by rapamycin in prostate cancer cell proliferation and survival. Oncogene 27, 7106–7117 (2008).
Liao, X. et al. Androgen stimulates matrix metalloproteinase-2 expression in human prostate cancer. Endocrinology 144, 1656–1663 (2003).
Chuan, Y.-C. et al. Androgen induction of prostate cancer cell invasion is mediated by ezrin. J. Biol. Chem. 281, 29938–29948 (2006).
Hara, T., Miyazaki, H., Lee, A., Tran, C. P. & Reiter, R. E. Androgen receptor and invasion in prostate cancer. Cancer Res. 68, 1128–1135 (2008).
Teh, M.-T. et al. FOXM1 induces a global methylation signature that mimics the cancer epigenome in head and neck squamous cell carcinoma. PLoS One 7, e34329 (2012).
Tsouko, E. et al. Regulation of the pentose phosphate pathway by an androgen receptor–mTOR-mediated mechanism and its role in prostate cancer cell growth. Oncogenesis 3, e103–e103 (2014).
Choi, S. Y. C. et al. The MCT4 gene: a novel, potential target for therapy of advanced prostate cancer. Clin. Cancer Res. 22, 2721–2733 (2016).
Koundouros, N. & Poulogiannis, G. Reprogramming of fatty acid metabolism in cancer. Br. J. Cancer 122, 4–22 (2020).
Poulose, N., Mills, I. G. & Steele, R. E. The impact of transcription on metabolism in prostate and breast cancers. Endocr. Relat. Cancer 25, R435–R452 (2018).
Ono, M. et al. [14C] fluciclovine (alias anti-[14C] FACBC) uptake and ASCT2 expression in castration-resistant prostate cancer cells. Nucl. Med. Biol. 42, 887–892 (2015).
White, M. A. et al. Glutamine transporters are targets of multiple oncogenic signaling pathways in prostate cancer. Mol. Cancer Res. 15, 1017–1028 (2017).
Corbin, J. M. & Ruiz-Echevarría, M. J. One-carbon metabolism in prostate cancer: the role of androgen signaling. Int. J. Mol. Sci. 17, 1208 (2016).
Shukla-Dave, A. et al. Ornithine decarboxylase is sufficient for prostate tumorigenesis via androgen receptor signaling. Am. J. Pathol. 186, 3131–3145 (2016).
Polkinghorn, W. R. et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov. 3, 1245–1253 (2013).
Sandhu, S. et al. Poly (ADP-ribose) polymerase (PARP) inhibitors for the treatment of advanced germline BRCA2 mutant prostate cancer. Ann. Oncol. 24, 1416–1418 (2013).
Goodwin, J. F. et al. A hormone–DNA repair circuit governs the response to genotoxic insult. Cancer Discov. 3, 1254–1271 (2013).
Guo, Z. et al. Regulation of androgen receptor activity by tyrosine phosphorylation. Cancer Cell 10, 309–319 (2006).
Liu, Y. et al. Dasatinib inhibits site-specific tyrosine phosphorylation of androgen receptor by Ack1 and Src kinases. Oncogene 29, 3208–3216 (2010).
Mellinghoff, I. K. et al. HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell 6, 517–527 (2004).
Seaton, A. et al. Interleukin-8 signaling promotes androgen-independent proliferation of prostate cancer cells via induction of androgen receptor expression and activation. Carcinogenesis 29, 1148–1156 (2008).
Fan, W. et al. Insulin-like growth factor 1/insulin signaling activates androgen signaling through direct interactions of Foxo1 with androgen receptor. J. Biol. Chem. 282, 7329–7338 (2007).
Migliaccio, A. et al. Steroid-induced androgen receptor–oestradiol receptor β–Src complex triggers prostate cancer cell proliferation. EMBO J. 19, 5406–5417 (2000).
Oliver, V. L., Poulios, K., Ventura, S. & Haynes, J. M. A novel androgen signalling pathway uses dihydrotestosterone, but not testosterone, to activate the EGF receptor signalling cascade in prostate stromal cells. Br. J. Pharmacol. 170, 592–601 (2013).
Sun, Y. H., Gao, X., Tang, Y. J., Xu, C. L. & Wang, L. H. Androgens induce increases in intracellular calcium via a G protein-coupled receptor in LNCaP prostate cancer cells. J. Androl. 27, 671–678 (2006).
ClinicalTrials.gov. US National Library of Medicine. https://ClinicalTrials.gov/show/NCT01084759 (2016).
ClinicalTrials.gov. US National Library of Medicine. https://ClinicalTrials.gov/show/NCT01750398 (2016).
ClinicalTrials.gov. US National Library of Medicine. https://ClinicalTrials.gov/show/NCT02286921 (2020).
ClinicalTrials.gov. US National Library of Medicine. https://ClinicalTrials.gov/show/NCT03554317 (2022).
ClinicalTrials.gov. US National Library of Medicine. https://ClinicalTrials.gov/show/NCT03516812 (2022).
Acknowledgements
S.K. is partly supported by the W81XWH1910724, 1R01CA243184 and PCF Challenge awards. R.K. is supported by the W81XWH2210118 and PCF Young Investigator Award 21YOUN22. L.A.S. is supported by W81XWH2010079 and Johns Hopkins University Clinician-Scientist Award.
Author information
Authors and Affiliations
Contributions
R.K., L.A.S. and S.K. researched data for the article. All authors contributed substantially to discussion of the content. R.K., L.A.S. and S.K. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
All the authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Urology thanks Stephen Plymate, Alessandro Tafuri and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, R., Sena, L.A., Denmeade, S.R. et al. The testosterone paradox of advanced prostate cancer: mechanistic insights and clinical implications. Nat Rev Urol 20, 265–278 (2023). https://doi.org/10.1038/s41585-022-00686-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-022-00686-y
This article is cited by
-
Bipolar androgen therapy plus nivolumab for patients with metastatic castration-resistant prostate cancer: the COMBAT phase II trial
Nature Communications (2024)
-
HP1α promotes the progression of prostate cancer
Molecular Biology Reports (2023)