Abstract
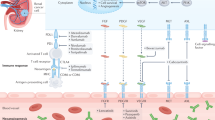
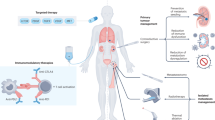
Localized renal cell carcinoma (RCC) is primarily managed with nephrectomy, which is performed with curative intent. However, disease recurs in ~20% of patients. Treatment with adjuvant therapies is used after surgery with the intention of curing additional patients by disrupting the establishment, maturation or survival of micrometastases, processes collectively referred to as the metastatic cascade. Immune checkpoint inhibitors and vascular endothelial growth factor receptor (VEGFR)-targeting tyrosine kinase inhibitors (TKIs) have shown efficacy in the treatment of metastatic RCC, increasing the interest in the utility of these agents in the adjuvant setting. Pembrolizumab, an inhibitor of the immune checkpoint PD1, is now approved by the FDA and is under review by European regulatory agencies for the adjuvant treatment of patients with localized resected clear cell RCC based on the results of the KEYNOTE-564 trial. However, the optimal use of immunotherapy and VEGFR-targeting TKIs for adjuvant treatment of RCC is not completely understood. These agents disrupt the metastatic cascade at multiple steps, providing biological rationale for further investigating the applications of these therapeutics in the adjuvant setting. Clinical trials to evaluate adjuvant therapeutics in RCC are ongoing, and clinical considerations must guide the practical use of immunotherapy and TKI agents for the adjuvant treatment of localized resected RCC.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Amin, M. B. et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 67, 93–99 (2017).
National Comprehensive Cancer Network. Kidney cancer (v4.2022). NCCN https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf (2022).
Escudier, B. et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 30, 706–720 (2019).
Kattan, M. W., Reuter, V., Motzer, R. J., Katz, J. & Russo, P. A postoperative prognostic nomogram for renal cell carcinoma. J. Urol. 166, 63–67 (2001).
Cindolo, L. et al. Comparison of predictive accuracy of four prognostic models for nonmetastatic renal cell carcinoma after nephrectomy: a multicenter European study. Cancer 104, 1362–1371 (2005).
Gong, J., Maia, M. C., Dizman, N., Govindarajan, A. & Pal, S. K. Metastasis in renal cell carcinoma: biology and implications for therapy. Asian J. Urol. 3, 286–292 (2016).
Sharma, P. & Allison, J. P. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 161, 205–214 (2015).
Seidel, J. A., Otsuka, A. & Kabashima, K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front. Oncol. https://doi.org/10.3389/fonc.2018.00086 (2018).
Fitzgerald, K. N. & Lee, C.-H. Personalizing first-line management of metastatic renal cell carcinoma: leveraging current and novel therapeutic options. J. Natl Compr. Cancer Netw. https://doi.org/10.6004/jnccn.2022.7003 (2022).
Rini, B. I. VEGF-targeted therapy in metastatic renal cell carcinoma. Oncologist 10, 191–197 (2005).
Rini, B. I. & Small, E. J. Biology and clinical development of vascular endothelial growth factor-targeted therapy in renal cell carcinoma. J. Clin. Oncol. 23, 1028–1043 (2005).
Karaman, S., Leppänen, V.-M. & Alitalo, K. Vascular endothelial growth factor signaling in development and disease. Development https://doi.org/10.1242/dev.151019 (2018).
Motzer, R. et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N. Engl. J. Med. 384, 1289–1300 (2021).
Motzer, R. J. et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 378, 1277–1290 (2018).
Rini, B. I. et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 380, 1116–1127 (2019).
Choueiri, T. K. et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 384, 829–841 (2021).
Motzer, R. J. et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 380, 1103–1115 (2019).
Choueiri, T. K. et al. Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N. Engl. J. Med. 385, 683–694 (2021).
Massari, F. et al. Adjuvant tyrosine kinase inhibitors in treatment of renal cell carcinoma: a meta-analysis of available clinical trials. Clin. Genitourin. Cancer 17, e339–e344 (2019).
Fidler, I. J. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer 3, 453–458 (2003).
Gupta, G. P. & Massagué, J. Cancer metastasis: building a framework. Cell 127, 679–695 (2006).
Ganesh, K. & Massagué, J. Targeting metastatic cancer. Nat. Med. 27, 34–44 (2021).
Borriello, L. et al. The role of the tumor microenvironment in tumor cell intravasation and dissemination. Eur. J. Cell Biol. 99, 151098 (2020).
Chiang, S. P. H., Cabrera, R. M. & Segall, J. E. Tumor cell intravasation. Am. J. Physiol. Cell Physiol. 311, C1–C14 (2016).
Perea Paizal, J., Au, S. H. & Bakal, C. Squeezing through the microcirculation: survival adaptations of circulating tumour cells to seed metastasis. Br. J. Cancer 124, 58–65 (2021).
Liu, Q., Liao, Q. & Zhao, Y. Myeloid-derived suppressor cells (MDSC) facilitate distant metastasis of malignancies by shielding circulating tumor cells (CTC) from immune surveillance. Med. Hypotheses 87, 34–39 (2016).
Wang, W.-C. et al. Survival mechanisms and influence factors of circulating tumor cells. BioMed. Res. Int. 2018, 6304701 (2018).
Sceneay, J., Smyth, M. J. & Möller, A. The pre-metastatic niche: finding common ground. Cancer Metastasis Rev. 32, 449–464 (2013).
Guise, T. Examining the metastatic niche: targeting the microenvironment. Semin. Oncol. 37, S2–S14 (2010).
Almholt, K. & Johnsen, M. Stromal cell involvement in cancer. Recent Results Cancer Res. 162, 31–42 (2003).
Kuczynski, E. A., Vermeulen, P. B., Pezzella, F., Kerbel, R. S. & Reynolds, A. R. Vessel co-option in cancer. Nat. Rev. Clin. Oncol. 16, 469–493 (2019).
Park, S.-Y. & Nam, J.-S. The force awakens: metastatic dormant cancer cells. Exp. Mol. Med. 52, 569–581 (2020).
Phan, T. G. & Croucher, P. I. The dormant cancer cell life cycle. Nat. Rev. Cancer 20, 398–411 (2020).
Vinay, D. S. et al. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 35, S185–S198 (2015).
Perego, M. et al. Reactivation of dormant tumor cells by modified lipids derived from stress-activated neutrophils. Sci. Transl. Med. 12, eabb5817 (2020).
Baeriswyl, V. & Christofori, G. The angiogenic switch in carcinogenesis. Semin. Cancer Biol. 19, 329–337 (2009).
Klein, C. A. Parallel progression of primary tumours and metastases. Nat. Rev. Cancer 9, 302–312 (2009).
Marcus, S. G. et al. Regression of metastatic renal cell carcinoma after cytoreductive nephrectomy. J. Urol. 150, 463–466 (1993).
Snow, R. M. & Schellhammer, P. F. Spontaneous regression of metastatic renal cell carcinoma. Urology 20, 177–181 (1982).
Bhindi, B. et al. Systematic review of the role of cytoreductive nephrectomy in the targeted therapy era and beyond: an individualized approach to metastatic renal cell carcinoma. Eur. Urol. 75, 111–128 (2019).
Okazaki, A. et al. Spontaneous regression of multiple pulmonary metastases accompanied by normalization of serum immune markers following cytoreductive nephrectomy in a patient with clear-cell renal cell carcinoma. IJU Case Rep. 4, 95–99 (2021).
Saleh, F. et al. Direct evidence on the immune-mediated spontaneous regression of human cancer: an incentive for pharmaceutical companies to develop a novel anti-cancer vaccine. Curr. Pharm. Des. 11, 3531–3543 (2005).
Kucerova, P. & Cervinkova, M. Spontaneous regression of tumour and the role of microbial infection – possibilities for cancer treatment. Anticancer Drugs 27, 269–277 (2016).
Franses, J. W. et al. Spontaneous immune-mediated regression of hepatocellular carcinoma with high tumor mutational burden. JCO Precis. Oncol. https://doi.org/10.1200/PO.21.00092 (2021).
Jessy, T. Immunity over inability: the spontaneous regression of cancer. J. Nat. Sci. Biol. Med. 2, 43–49 (2011).
Motzer, R. J. et al. Survival outcomes and independent response assessment with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma: 42-month follow-up of a randomized phase 3 clinical trial. J. Immunother. Cancer 8, e000891 (2020).
Marasco, M. et al. Molecular mechanism of SHP2 activation by PD-1 stimulation. Sci. Adv. 6, eaay4458 (2020).
Hui, E. et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 355, 1428–1433 (2017).
Freeman, G. J. et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 192, 1027–1034 (2000).
McDermott, D. F. & Atkins, M. B. PD-1 as a potential target in cancer therapy. Cancer Med. 2, 662–673 (2013).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Janjigian, Y. Y., Wolchok, J. D. & Ariyan, C. E. Eradicating micrometastases with immune checkpoint blockade: strike while the iron is hot. Cancer Cell 39, 738–742 (2021).
Rzhevskiy, A. et al. Emerging role of circulating tumor cells in immunotherapy. Theranostics 11, 8057–8075 (2021).
Zhou, Y., Han, M. & Gao, J. Prognosis and targeting of pre-metastatic niche. J. Control. Rel. 325, 223–234 (2020).
Risson, E., Nobre, A. R., Maguer-Satta, V. & Aguirre-Ghiso, J. A. The current paradigm and challenges ahead for the dormancy of disseminated tumor cells. Nat. Cancer 1, 672–680 (2020).
Labani-Motlagh, A., Ashja-Mahdavi, M. & Loskog, A. The tumor microenvironment: a milieu hindering and obstructing antitumor immune responses. Front. Immunol. https://doi.org/10.3389/fimmu.2020.00940 (2020).
Dammeijer, F. et al. The PD-1/PD-L1-checkpoint restrains T cell immunity in tumor-draining lymph nodes. Cancer Cell 38, 685–700 (2020).
Dye, E. The antimetastatic function of concomitant antitumor immunity. II. Evidence that the generation of Ly-1+ 2+ effector T cells temporarily causes the destruction of already disseminated tumor cells. J. Immunol. 136, 1510–1515 (1986).
Eyles, J. et al. Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. J. Clin. Invest. 120, 2030–2039 (2010).
Vetsika, E. K. et al. A circulating subpopulation of monocytic myeloid-derived suppressor cells as an independent prognostic/predictive factor in untreated non-small lung cancer patients. J. Immunol. Res. https://doi.org/10.1155/2014/659294 (2014).
Gambichler, T. et al. A brief communication on circulating PD-1-positive T-regulatory lymphocytes in melanoma patients undergoing adjuvant immunotherapy with nivolumab. J. Immunother. 42, 265–268 (2019).
Woods, D. M. et al. Decreased suppression and increased phosphorylated STAT3 in regulatory T cells are associated with benefit from adjuvant PD-1 blockade in resected metastatic melanoma. Clin. Cancer Res. 24, 6236–6247 (2018).
Coffelt, S. B. et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 522, 345–348 (2015).
Wculek, S. & Malanchi, I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature 528, 413–417 (2015).
Tian, W. et al. Leukotrienes in tumor-associated inflammation. Front. Pharmacol. https://doi.org/10.3389/fphar.2020.01289 (2020).
Wang, T. T. et al. Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-L1 pathway. Gut 66, 1900–1911 (2017).
Eruslanov, E. B. et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J. Clin. Invest. 124, 5466–5480 (2014).
Lin, E. Y., Nguyen, A. V., Russell, R. G. & Pollard, J. W. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J. Exp. Med. 193, 727–740 (2001).
Lin, E. Y. et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 66, 11238–11246 (2006).
Hiratsuka, S. et al. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell 2, 289–300 (2002).
Gordon, S. et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 545, 495–499 (2017).
Pollard, J. W. Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer 4, 71–78 (2004).
Tallón de Lara, P. et al. CD39+PD-1+CD8+ T cells mediate metastatic dormancy in breast cancer. Nat. Commun. 12, 769 (2021).
Sharpe, A. H. & Pauken, K. E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 18, 153–167 (2018).
Haas, N. B. et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet 387, 2008–2016 (2016).
Gross-Goupil, M. et al. Axitinib versus placebo as an adjuvant treatment of renal cell carcinoma: results from the phase III, randomized ATLAS trial. Ann. Oncol. 29, 2371–2378 (2018).
Motzer, R. J. et al. Adjuvant sunitinib for high-risk renal cell carcinoma after nephrectomy: subgroup analyses and updated overall survival results. Eur. Urol. 73, 62–68 (2018).
Motzer, R. J. et al. Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with localized or locally advanced renal cell carcinoma. J. Clin. Oncol. 35, 3916–3923 (2017).
Ravaud, A. et al. Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N. Engl. J. Med. 375, 2246–2254 (2016).
Ferrara, N., Gerber, H.-P. & LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 9, 669–676 (2003).
Yang, J., Yan, J. & Liu, B. Targeting VEGF/VEGFR to modulate antitumor immunity. Front. Immunol. https://doi.org/10.3389/fimmu.2018.00978 (2018).
Bhattacharya, R. et al. Intracrine VEGF signalling mediates colorectal cancer cell migration and invasion. Br. J. Cancer 117, 848–855 (2017).
Islam, M. R., Jones, S. J., Macluskey, M. & Ellis, I. R. Is there a pAkt between VEGF and oral cancer cell migration? Cell. Signal. 26, 1294–1302 (2014).
Timoshenko, A., Rastogi, S. & Lala, P. Migration-promoting role of VEGF-C and VEGF-C binding receptors in human breast cancer cells. Br. J. cancer 97, 1090–1098 (2007).
Kim, K. J. et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 362, 841–844 (1993).
Huang, D. et al. Sunitinib acts primarily on tumor endothelium rather than tumor cells to inhibit the growth of renal cell carcinoma. Cancer Res. 70, 1053–1062 (2010).
Bhattacharya, R. et al. Intracrine VEGF signaling mediates the activity of prosurvival pathways in human colorectal cancer cells. Cancer Res. 76, 3014–3024 (2016).
Zheng, B. et al. VEGFR2 promotes metastasis and PD-L2 expression of human osteosarcoma cells by activating the STAT3 and RhoA-ROCK-LIMK2 pathways. Front. Oncol. 10, 1865 (2020).
Wang, W., Eddy, R. & Condeelis, J. The cofilin pathway in breast cancer invasion and metastasis. Nat. Rev. Cancer 7, 429–440 (2007).
Yamaguchi, H. & Condeelis, J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim. Biophys. Acta 1773, 642–652 (2007).
Haspel, H. C., Scicli, G. M., McMahon, G. & Scicli, A. G. Inhibition of vascular endothelial growth factor-associated tyrosine kinase activity with SU5416 blocks sprouting in the microvascular endothelial cell spheroid model of angiogenesis. Microvasc. Res. 63, 304–315 (2002).
López-Soto, A., Gonzalez, S., Smyth, M. J. & Galluzzi, L. Control of metastasis by NK cells. Cancer Cell 32, 135–154 (2017).
Tallón de Lara, P., Castañón, H., Sterpi, M. & van den Broek, M. Antimetastatic defense by CD8+ T cells. Trends Cancer https://doi.org/10.1016/j.trecan.2021.10.006 (2021).
Gavalas, N. G. et al. VEGF directly suppresses activation of T cells from ascites secondary to ovarian cancer via VEGF receptor type 2. Br. J. Cancer 107, 1869–1875 (2012).
Kaur, S. et al. CD47 signaling regulates the immunosuppressive activity of VEGF in T cells. J. Immunol. 193, 3914–3924 (2014).
Hage, C. et al. Sorafenib induces pyroptosis in macrophages and triggers natural killer cell-mediated cytotoxicity against hepatocellular carcinoma. Hepatology 70, 1280–1297 (2019).
Ruan, G.-X. & Kazlauskas, A. VEGF-A engages at least three tyrosine kinases to activate PI3K/Akt. Cell Cycle 11, 2047–2048 (2012).
Liu, L. et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 66, 11851–11858 (2006).
Ribatti, D. Judah Folkman, a pioneer in the study of angiogenesis. Angiogenesis 11, 3–10 (2008).
Folkman, J. Anti-angiogenesis: new concept for therapy of solid tumors. Ann. Surg. 175, 409–416 (1972).
Bergers, G. & Benjamin, L. E. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer 3, 401–410 (2003).
Pezzella, F. et al. Non-small-cell lung carcinoma tumor growth without morphological evidence of neo-angiogenesis. Am. J. Pathol. 151, 1417–1423 (1997).
Holash, J., Wiegand, S. J. & Yancopoulos, G. D. New model of tumor angiogenesis: dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene 18, 5356–5362 (1999).
Döme, B., Paku, S., Somlai, B. & Tímár, J. Vascularization of cutaneous melanoma involves vessel co-option and has clinical significance. J. Pathol. 197, 355–362 (2002).
Holash, J. et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 284, 1994–1998 (1999).
Küsters, B. et al. Vascular endothelial growth factor-A(165) induces progression of melanoma brain metastases without induction of sprouting angiogenesis. Cancer Res. 62, 341–345 (2002).
Döme, B., Hendrix, M. J. C., Paku, S., Tóvári, J. & Tímár, J. Alternative vascularization mechanisms in cancer: pathology and therapeutic implications. Am. J. Pathol. 170, 1–15 (2007).
Lee, Y.-J. et al. Differential effects of VEGFR-1 and VEGFR-2 inhibition on tumor metastases based on host organ environment. Cancer Res. 70, 8357–8367 (2010).
Wada, J. et al. The contribution of vascular endothelial growth factor to the induction of regulatory T-cells in malignant effusions. Anticancer. Res. 29, 881–888 (2009).
Dikov, M. M. et al. Differential roles of vascular endothelial growth factor receptors 1 and 2 in dendritic cell differentiation. J. Immunol. 174, 215–222 (2005).
Du Four, S. et al. Axitinib increases the infiltration of immune cells and reduces the suppressive capacity of monocytic MDSCs in an intracranial mouse melanoma model. Oncoimmunology 4, e998107 (2015).
Ryan, C. W. et al. EVEREST: everolimus for renal cancer ensuing surgical therapy — a phase III study (SWOG S0931, NCT01120249) [abstract]. J. Clin. Oncol. 40, LBA4500 (2022).
Hoefflin, R. et al. HIF-1α and HIF-2α differently regulate tumour development and inflammation of clear cell renal cell carcinoma in mice. Nat. Commun. 11, 4111 (2020).
Linehan, W. M. & Ricketts, C. J. The cancer genome atlas of renal cell carcinoma: findings and clinical implications. Nat. Rev. Urol. 16, 539–552 (2019).
Voss, M. H., Molina, A. M. & Motzer, R. J. mTOR inhibitors in advanced renal cell carcinoma. Hematol./Oncol. Clin. 25, 835–852 (2011).
Courcier, J. et al. Carbonic anhydrase IX in renal cell carcinoma, implications for disease management. Int. J. Mol. Sci. 21, 7146 (2020).
Chamie, K. et al. Adjuvant weekly girentuximab following nephrectomy for high-risk renal cell carcinoma: the ARISER randomized clinical trial. JAMA Oncol. 3, 913–920 (2017).
Motzer, R. J. et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 373, 1803–1813 (2015).
McDermott, D. F. et al. Open-label, single-arm, phase II study of pembrolizumab monotherapy as first-line therapy in patients with advanced non-clear cell renal cell carcinoma. J. Clin. Oncol. 39, 1029–1039 (2018).
US Food and Drug Administration. FDA approves pembrolizumab for adjuvant treatment of renal cell carcinoma. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-adjuvant-treatment-renal-cell-carcinoma (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03055013 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03024996 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03138512 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03288532 (2020).
Harshman, L. C. et al. PROSPER: A phase III randomized study comparing perioperative nivolumab (nivo) versus observation in patients with localized renal cell carcinoma (RCC) undergoing nephrectomy (ECOG-ACRIN 8143) [abstract]. J. Clin. Oncol. 37 (Suppl. 7), TPS684 (2019).
Allaf, M. et al. Phase III randomized study comparing perioperative nivolumab (nivo) versus observation in patients (Pts) with renal cell carcinoma (RCC) undergoing nephrectomy (PROSPER, ECOG-ACRIN EA8143), a National Clinical Trials Network trial [abstract LBA67]. Ann. Oncol. 33 (Suppl. 7), 1432–1433 (2022).
Bex, A. et al. IMmotion010: Efficacy and safety from the phase III study of atezolizumab (atezo) vs placebo (pbo) as adjuvant therapy in patients with renal cell carcinoma (RCC) at increased risk of recurrence after resection [abstract LBA66]. Ann. Oncol. 33 (Suppl. 7), 1431–1432 (2022).
Motzer, R. J. et al. Adjuvant nivolumab plus ipilimumab (NIVO+IPI) vs placebo (PBO) for localized renal cell carcinoma (RCC) at high risk of relapse after nephrectomy: Results from the randomized, phase III CheckMate 914 trial [abstract LBA4]. Ann. Oncol. 33 (Suppl. 7), 1430 (2022).
Allen, B. M. et al. Systemic dysfunction and plasticity of the immune macroenvironment in cancer models. Nat. Med. 26, 1125–1134 (2020).
Danna, E. A. et al. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res. 64, 2205–2211 (2004).
Lam, J. S. et al. Postoperative surveillance protocol for patients with localized and locally advanced renal cell carcinoma based on a validated prognostic nomogram and risk group stratification system. J. Urol. 174, 466–472 (2005).
Haas, N. B. et al. Adjuvant treatment for high-risk clear cell renal cancer: updated results of a high-risk subset of the ASSURE randomized trial. JAMA Oncol. 3, 1249–1252 (2017).
Haas, N. et al. Dose analysis of ASSURE (E2805): adjuvant sorafenib or sunitinib for unfavorable renal carcinoma, an ECOG-ACRIN-led, NCTN phase 3 trial [abstract]. J. Clin. Oncol. 33 (Suppl. 15), 4508 (2015).
Edge, S. B. & Compton, C. C. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 17, 1471–1474 (2010).
Leibovich, B. C. et al. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer 97, 1663–1671 (2003).
Eisen, T. et al. Adjuvant sorafenib for renal cell carcinoma at intermediate or high risk of relapse: results from the SORCE randomized phase III intergroup trial. J. Clin. Oncol. 38, 4064–4075 (2020).
US Food and Drug Administration. FDA approves sunitinib malate for adjuvant treatment of renal cell carcinoma FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-sunitinib-malate-adjuvant-treatment-renal-cell-carcinoma (2018).
US Food and Drug Administration. Project optimus. FDA https://www.fda.gov/about-fda/oncology-center-excellence/project-optimus (2022).
Choueiri, T. K. et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 373, 1814–1823 (2015).
Rini, B. I. et al. Axitinib in metastatic renal cell carcinoma: results of a pharmacokinetic and pharmacodynamic analysis. J. Clin. Pharmacol. 53, 491–504 (2013).
Roviello, G. et al. Lenvatinib for the treatment of renal cell carcinoma. Expert. Opin. Invest. Drugs 27, 507–512 (2018).
Postow, M. A., Sidlow, R. & Hellmann, M. D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 378, 158–168 (2018).
Correa, A. F. et al. Predicting renal cancer recurrence: defining limitations of existing prognostic models with prospective trial-based validation. J. Clin. Oncol. 37, 2062–2071 (2019).
Agrawal, S. et al. Eligibility and radiologic assessment for adjuvant clinical trials in kidney cancer. JAMA Oncol. 6, 133–141 (2020).
Oza, B. et al. External validation of the 2003 Leibovich prognostic score in patients randomly assigned to SORCE, an international phase III trial of adjuvant sorafenib in renal cell cancer. J. Clin. Oncol. 40, 1772–1782 (2022).
Brookman-Amissah, S. et al. Impact of clinical variables on predicting disease-free survival of patients with surgically resected renal cell carcinoma. BJU Int. 103, 1375–1380 (2009).
Hakimi, A. A. et al. Transcriptomic profiling of the tumor microenvironment reveals distinct subgroups of clear cell renal cell cancer: data from a randomized phase III trial. Cancer Discov. 9, 510–525 (2019).
Beuselinck, B. et al. Molecular subtypes of clear cell renal cell carcinoma are associated with sunitinib response in the metastatic setting. Clin. Cancer Res. 21, 1329–1339 (2015).
Brooks, S. A. et al. ClearCode34: a prognostic risk predictor for localized clear cell renal cell carcinoma. Eur. Urol. 66, 77–84 (2014).
Askeland, E. J. et al. Cell cycle progression score predicts metastatic progression of clear cell renal cell carcinoma after resection. Cancer Biomark. 15, 861–867 (2015).
Ghatalia, P. et al. Prognostic impact of immune gene expression signature and tumor infiltrating immune cells in localized clear cell renal cell carcinoma. J. Immunother. Cancer 7, 139 (2019).
Turajlic, S. et al. Tracking cancer evolution reveals constrained routes to metastases: TRACERx renal. Cell 173, 581–594 (2018).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT05239533 (2022).
Shuch, B. P. et al. TLX250-CDx, multi-center phase III trial for PET/CT imaging of clear cell kidney cancer in International Kidney Cancer Symposium (IKCS) 2021 (IKCS, 2021).
George, D. J. et al. Immune biomarkers predictive for disease-free survival with adjuvant sunitinib in high-risk locoregional renal cell carcinoma: from randomized phase III S-TRAC study. Clin. Cancer Res. 24, 1554–1561 (2018).
Choueiri, T. K. et al. KEYNOTE-564: A phase 3, randomized, double blind, trial of pembrolizumab in the adjuvant treatment of renal cell carcinoma [abstract]. J. Clin. Oncol. 36 (Suppl. 15), TPS4599 (2018).
European Medicines Agency. Appendix 1 to the guideline on the evaluation of anticancer medicinal products in man. European Medicines Agency https://www.ema.europa.eu/en/documents/scientific-guideline/appendix-1-guideline-evaluation-anticancer-medicinal-products-man-methodological-consideration-using_en.pdf (2012).
US Food and Drug Administration. Renal cell carcinoma: developing drugs and biologics for adjuvant treatment: guidance for industry. FDA https://www.fda.gov/media/159510/download (2022).
Harshman, L. C. et al. Evaluation of disease-free survival as an intermediate metric of overall survival in patients with localized renal cell carcinoma: a trial-level meta-analysis. Cancer 124, 925–933 (2018).
Woodford, R. G. et al. The validity of progression‐free survival 2 as a surrogate trial end point for overall survival. Cancer 128, 1449–1457 (2022).
Au, L. et al. Determinants of anti-PD-1 response and resistance in clear cell renal cell carcinoma. Cancer Cell 39, 1497–1518.e11 (2021).
Jonasch, E. et al. Belzutifan for renal cell carcinoma in von Hippel–Lindau disease. N. Engl. J. Med. 385, 2036–2046 (2021).
US Food and Drug Administrstion. FDA approves belzutifan for cancers associated with von Hippel-Lindau disease FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-belzutifan-cancers-associated-von-hippel-lindau-disease (2022).
European Medicines Agency. EU/3/20/2324: Orphan designation for the treatment of von Hippel-Lindau disease. European Medicines Agency https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu3202324 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04522323 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04696731 (2022).
Lee, C.-H. et al. Phase II trial of cabozantinib plus nivolumab in patients with non-clear-cell renal cell carcinoma and genomic correlates. J. Clin. Oncol. 40, 2333–2341 (2022).
Gupta, R. et al. Clinical activity of ipilimumab plus nivolumab in patients with metastatic non-clear cell renal cell carcinoma. Clin. Genitourin. Cancer 18, 429–435 (2020).
Koshkin, V. S. et al. Clinical activity of nivolumab in patients with non-clear cell renal cell carcinoma. J. Immunother. Cancer 6, 9 (2018).
Karam, J. A. et al. Phase 2 trial of neoadjuvant axitinib in patients with locally advanced nonmetastatic clear cell renal cell carcinoma. Eur. Urol. 66, 874–880 (2014).
Bex, A. et al. Efficacy, safety, and biomarker analysis of neoadjuvant avelumab/axitinib in patients (pts) with localized renal cell carcinoma (RCC) who are at high risk of relapse after nephrectomy (NeoAvAx) [abstract]. J. Clin. Oncol. 40 (Suppl. 6), 289 (2022).
US National Library of Medicine. Support materials. ClinicalTrials.gov https://clinicaltrials.gov/ct2/manage-recs/resources#DataElement (2022).
Cindolo, L. et al. A preoperative clinical prognostic model for non-metastatic renal cell carcinoma. BJU Int. 92, 901–905 (2003).
Karakiewicz, P. I. et al. Multi-institutional validation of a new renal cancer-specific survival nomogram. J. Clin. Oncol. 25, 1316–1322 (2007).
Sorbellini, M. et al. A postoperative prognostic nomogram predicting recurrence for patients with conventional clear cell renal cell carcinoma. J. Urol. 173, 48–51 (2005).
Brookman-May, S. et al. Features associated with recurrence beyond 5 years after nephrectomy and nephron-sparing surgery for renal cell carcinoma: development and internal validation of a risk model (PRELANE score) to predict late recurrence based on a large multicenter database (CORONA/SATURN project). Eur. Urol. 64, 472–477 (2013).
Frank, I. et al. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J. Urol. 168, 2395–2400 (2002).
Zisman, A. et al. Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. J. Clin. Oncol. 20, 4559–4566 (2002).
Yaycioglu, O. et al. Prognostic assessment of nonmetastatic renal cell carcinoma: a clinically based model. Urology 58, 141–145 (2001).
Sobin, L. H. & Fleming, I. D. TNM classification of malignant tumors, fifth edition (1997). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer 80, 1803–1804 (1997).
Wittekind, C., Compton, C. C., Greene, F. L. & Sobin, L. H. TNM residual tumor classification revisited. Cancer 94, 2511–2516 (2002).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02597322 (2017).
Zhang, X. & Xu, W. Neutrophils diminish T-cell immunity to foster gastric cancer progression: the role of GM-CSF/PD-L1/PD-1 signalling pathway. Gut 66, 1878–1880 (2017).
Acknowledgements
The authors thank the National Institute of Health Clinical and Translational Science Awards Program, grant number UL1TR00457. They are also grateful for the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant P30 CA008748.
Author information
Authors and Affiliations
Contributions
K.N.F. and R.J.M. researched data for the article. All authors contributed substantially to discussion of the content. All authors wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
R.J.M. reports grants from Bristol Myers Squibb, grants and personal fees from Pfizer, Novartis, Eisai, Exelixis, Merck and Genentech/Roche, and personal fees from Lilly, Incyte, EMD Serono Research and Development Institute, AVEO Pharmaceuticals and Takeda. C.-H.L reports honoraria from Intellisphere, Research to Practice and AiCME, consulting or advisory role with Amgen, Bristol Myers Squibb, Eisai, Exelixis, Merck and Pfizer/EMD Serono, institutional research funding from Bristol Myers Squibb, Calithera Biosciences, Eisai, Exelixis, Lilly, Merck and Pfizer, and travel/accommodation expenses from Calithera Biosciences and Eisai. K.N.F. declares no competing interests.
Peer review
Peer review information
Nature Reviews Urology thanks S. Brookman-May and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fitzgerald, K.N., Motzer, R.J. & Lee, CH. Adjuvant therapy options in renal cell carcinoma — targeting the metastatic cascade. Nat Rev Urol 20, 179–193 (2023). https://doi.org/10.1038/s41585-022-00666-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-022-00666-2