Abstract
Amyotrophic lateral sclerosis (ALS) is a complex neurodegenerative disease that is classically thought to impact the motor system. Over the past 20 years, research has started to consider the contribution of non-motor symptoms and features of the disease, and how they might affect ALS prognosis. Of the non-motor features of the disease, nutritional status (for example, malnutrition) and metabolic balance (for example, weight loss and hypermetabolism) have been consistently shown to contribute to more rapid disease progression and/or earlier death. Several complex cellular changes observed in ALS, including mitochondrial dysfunction, are also starting to be shown to contribute to bioenergetic failure. The resulting energy depletion in high energy demanding neurons makes them sensitive to apoptosis. Given that nutritional and metabolic stressors at the whole-body and cellular level can impact the capacity to maintain optimal function, these factors present avenues through which we can identify novel targets for treatment in ALS. Several clinical trials are now underway evaluating the effectiveness of modifying energy balance in ALS, making this article timely in reviewing the evidence base for metabolic and nutritional interventions.
Key points
-
Clinical and epidemiological evidence links metabolic alterations to amyotrophic lateral sclerosis (ALS) onset and progression; these metabolic defects precede motor symptoms, suggesting a causative role in ALS.
-
Although hypermetabolism is consistently observed in ALS, its causes and clinical relevance remain largely unknown; to address this, a consensus approach to identifying hypermetabolism in ALS is needed.
-
Exploring ALS metabolic dysregulation is key for optimizing patient care and analysing nutritional status, especially fat mass stability, is crucial for understanding energy homeostasis; imbalances might require energy intake interventions, orally or via gastrostomy.
-
Compounds that improve cellular bioenergetics exert neuroprotection in in vivo and in vitro ALS models, but most have failed in clinical trials or have provided modest benefit in people living with ALS.
-
Studies in mouse models and patient trials have demonstrated a potential therapeutic role for high-calorie diets in ALS, but optimal nutritional intervention parameters require further elucidation.
-
A personalized approach of bioenergetic enhancement with nutritional interventions might yield superior outcomes in ALS.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hardiman, O. et al. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Prim. 3, 17071, https://doi.org/10.1038/nrdp.2017.71 (2017).
Van Damme, P., Robberecht, W. & Van Den Bosch, L. Modelling amyotrophic lateral sclerosis: progress and possibilities. Dis. Model. Mech. 10, 537–549 (2017).
Renton, A. E., Chio, A. & Traynor, B. J. State of play in amyotrophic lateral sclerosis genetics. Nat. Neurosci. 17, 17–23 (2014).
Renton, A. E. et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72, 257–268 (2011).
Muller, K. et al. Comprehensive analysis of the mutation spectrum in 301 German ALS families. J. Neurol. Neurosurg. Psychiatry 89, 817–827 (2018).
Rosen, D. R. et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362, 59–62 (1993).
Kabashi, E. et al. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat. Genet. 40, 572–574 (2008).
Sreedharan, J. et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 319, 1668–1672 (2008).
Vance, C. et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science 323, 1208–1211 (2009).
McCann, E. P. et al. Evidence for polygenic and oligogenic basis of Australian sporadic amyotrophic lateral sclerosis. J. Med. Genet. 58, 87–95 (2020).
Mehta, P. R. et al. The impact of age on genetic testing decisions in amyotrophic lateral sclerosis. Brain 145, 4440–4447 (2022).
Shepheard, S. R. et al. Value of systematic genetic screening of patients with amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 92, 510–518 (2021).
Al-Chalabi, A. et al. Analysis of amyotrophic lateral sclerosis as a multistep process: a population-based modelling study. Lancet Neurol. 13, 1108–1113 (2014).
Chio, A. et al. The multistep hypothesis of ALS revisited: the role of genetic mutations. Neurology 91, e635–e642 (2018).
Filippini, T. et al. Environmental and occupational risk factors of amyotrophic lateral sclerosis: a population-based case-control study. Int. J. Environ. Res. Public Health https://doi.org/10.3390/ijerph17082882 (2020).
Bradley, W. G., Miller, R. X., Levine, T. D., Stommel, E. W. & Cox, P. A. Studies of environmental risk factors in amyotrophic lateral sclerosis (ALS) and a phase I clinical trial of l-serine. Neurotox. Res. 33, 192–198 (2018).
Wang, H. et al. Smoking and risk of amyotrophic lateral sclerosis: a pooled analysis of 5 prospective cohorts. Arch. Neurol. 68, 207–213 (2011).
Boddy, S. L. et al. The gut microbiome: a key player in the complexity of amyotrophic lateral sclerosis (ALS). BMC Med. https://doi.org/10.1186/s12916-020-01885-3 (2021).
Julian, T. H. et al. Physical exercise is a risk factor for amyotrophic lateral sclerosis: convergent evidence from Mendelian randomisation, transcriptomics and risk genotypes. EBioMedicine 68, 103397 (2021).
Desport, J. C. et al. Nutritional status is a prognostic factor for survival in ALS patients. Neurology 53, 1059–1063 (1999).
Kasarskis, E. J., Berryman, S., Vanderleest, J. G., Schneider, A. R. & McClain, C. J. Nutritional status of patients with amyotrophic lateral sclerosis: relation to the proximity of death. Am. J. Clin. Nutr. 63, 130–137 (1996).
O’Reilly, E. J. et al. Premorbid body mass index and risk of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Frontotemporal Degener. 14, 205–211 (2013).
Charcot, J. & Joffroy, A. Deux cas d’atrophie musculaire progressive avec lesions de la substance grise et de faisceaux antero-lateraux de la moelle epiniere. Arch. Physiol. Norm. Pathol. 1, 354–367 (1869).
Bensimon, G., Lacomblez, L. & Meininger, V. ALS/Riluzole Study Group. A controlled trial of riluzole in amyotrophic lateral sclerosis. N. Engl. J. Med. 330, 585–591 (1994).
Amyotrophic Lateral Sclerosis/Riluzole Study Group II et al. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Lancet 347, 1425–1431 (1996).
Abe, K. et al. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 16, 505–512 (2017).
Brooks, B. R. et al. Edaravone efficacy in amyotrophic lateral sclerosis with reduced forced vital capacity: post-hoc analysis of Study 19 (MCI186-19) [clinical trial NCT01492686]. PLoS ONE 17, e0258614 (2022).
Paganoni, S. et al. Effect of sodium phenylbutyrate/taurursodiol on tracheostomy/ventilation-free survival and hospitalisation in amyotrophic lateral sclerosis: long-term results from the CENTAUR trial. J. Neurol. Neurosurg. Psychiatry 93, 871–875 (2022).
Miller, T. M. et al. Trial of antisense oligonucleotide tofersen for SOD1 ALS. N. Engl. J. Med. 387, 1099–1110 (2022).
Vucic, S. et al. ALS is a multistep process in South Korean, Japanese, and Australian patients. Neurology 94, e1657–e1663 (2020).
Diekmann, K. et al. Impact of comorbidities and co-medication on disease onset and progression in a large German ALS patient group. J. Neurol. 267, 2130–2141 (2020).
Janse van Mantgem, M. R. et al. Prognostic value of weight loss in patients with amyotrophic lateral sclerosis: a population-based study. J. Neurol. Neurosurg. Psychiatry 91, 867–875 (2020).
Wei, Q. Q. et al. Early weight instability is associated with cognitive decline and poor survival in amyotrophic lateral sclerosis. Brain Res. Bull. 171, 10–15 (2021).
Li, J. Y. et al. Correlation of weight and body composition with disease progression rate in patients with amyotrophic lateral sclerosis. Sci. Rep. https://doi.org/10.1038/s41598-022-16229-9 (2022).
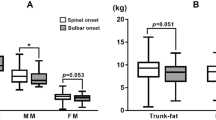
Kandler, K. et al. Phenotyping of the thoracic-onset variant of amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 93, 563–565 (2022).
Xia, K. et al. Mutation-specific metabolic profiles in presymptomatic amyotrophic lateral sclerosis. Eur. J. Neurol. 30, 87–95 (2023).
Nakayama, Y. et al. Body weight variation predicts disease progression after invasive ventilation in amyotrophic lateral sclerosis. Sci. Rep. 9, 12262 (2019).
Hesters, A. et al. Predictive factors for prognosis after gastrostomy placement in routine non-invasive ventilation users ALS patients. Sci. Rep. 10, 15117 (2020).
Marin, B. et al. Population-based evidence that survival in amyotrophic lateral sclerosis is related to weight loss at diagnosis. Neurodegener. Dis. 16, 225–234 (2016).
Marin, B. et al. Alteration of nutritional status at diagnosis is a prognostic factor for survival of amyotrophic lateral sclerosis patients. J. Neurol. Neurosurg. Psychiatry 82, 628–634 (2011).
Dardiotis, E. et al. Body mass index and survival from amyotrophic lateral sclerosis: a meta-analysis. Neurol. Clin. Pract. 8, 437–444 (2018).
Dorst, J. et al. Prognostic factors in ALS: a comparison between Germany and China. J. Neurol. 266, 1516–1525 (2019).
Witzel, S. et al. Fast versus slow disease progression in amyotrophic lateral sclerosis – clinical and genetic factors at the edges of the survival spectrum. Neurobiol. Aging 119, 117–126 (2022).
Dorst, J. et al. Patients with elevated triglyceride and cholesterol serum levels have a prolonged survival in amyotrophic lateral sclerosis. J. Neurol. 258, 613–617 (2011).
Dupuis, L. et al. Dyslipidemia is a protective factor in amyotrophic lateral sclerosis. Neurology 70, 1004–1009 (2008).
Chio, A. et al. Lower serum lipid levels are related to respiratory impairment in patients with ALS. Neurology 73, 1681–1685 (2009).
Tandan, R. et al. Body composition in amyotrophic lateral sclerosis subjects and its effect on disease progression and survival. Am. J. Clin. Nutr. 115, 1378–1392 (2022).
Lee, I. et al. Fat mass loss correlates with faster disease progression in amyotrophic lateral sclerosis patients: exploring the utility of dual-energy x-ray absorptiometry in a prospective study. PLoS ONE 16, e0251087 (2021).
Lindauer, E. et al. Adipose tissue distribution predicts survival in amyotrophic lateral sclerosis. PLoS ONE 8, e67783 (2013).
Paganoni, S., Deng, J., Jaffa, M., Cudkowicz, M. E. & Wills, A. M. Body mass index, not dyslipidemia, is an independent predictor of survival in amyotrophic lateral sclerosis. Muscle Nerve 44, 20–24 (2011).
Ngo, S. T. et al. Loss of appetite is associated with a loss of weight and fat mass in patients with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Frontotemporal Degener. 20, 497–505 (2019).
Moglia, C. et al. Early weight loss in amyotrophic lateral sclerosis: outcome relevance and clinical correlates in a population-based cohort. J. Neurol. Neurosurg. Psychiatry 90, 666–673 (2019).
Mezoian, T. et al. Loss of appetite in amyotrophic lateral sclerosis is associated with weight loss and decreased calorie consumption independent of dysphagia. Muscle Nerve 61, 230–234 (2020).
Mariosa, D. et al. Body mass index and amyotrophic lateral sclerosis: a study of US military veterans. Am. J. Epidemiol. 185, 362–371 (2017).
Westeneng, H. J. et al. Associations between lifestyle and amyotrophic lateral sclerosis stratified by C9orf72 genotype: a longitudinal, population-based, case-control study. Lancet Neurol. 20, 373–384 (2021).
Peter, R. S. et al. Life course body mass index and risk and prognosis of amyotrophic lateral sclerosis: results from the ALS registry Swabia. Eur. J. Epidemiol. 32, 901–908 (2017).
Nagel, G. et al. Adipokines, C-reactive protein and amyotrophic lateral sclerosis – results from a population- based ALS registry in Germany. Sci. Rep. 7, 4374 (2017).
Nagel, G. et al. Association of insulin-like growth factor 1 concentrations with risk for and prognosis of amyotrophic lateral sclerosis – results from the ALS registry Swabia. Sci. Rep. 10, 736 (2020).
Rosenbohm, A. et al. Association of serum retinol-binding protein 4 concentration with risk for and prognosis of amyotrophic lateral sclerosis. JAMA Neurol. 75, 600–607 (2018).
Gallo, V. et al. Prediagnostic body fat and risk of death from amyotrophic lateral sclerosis: the EPIC cohort. Neurology 80, 829–838 (2013).
Nakken, O., Meyer, H. E., Stigum, H. & Holmoy, T. High BMI is associated with low ALS risk: a population-based study. Neurology 93, e424–e432 (2019).
O’Reilly, E. J. et al. Prediagnostic body size and risk of amyotrophic lateral sclerosis death in 10 studies. Amyotroph. Lateral Scler. Frontotemporal Degener. 19, 396–406 (2018).
Mattsson, P., Lonnstedt, I., Nygren, I. & Askmark, H. Physical fitness, but not muscle strength, is a risk factor for death in amyotrophic lateral sclerosis at an early age. J. Neurol. Neurosurg. Psychiatry 83, 390–394 (2012).
Bjornevik, K. et al. Pre-diagnostic plasma lipid levels and the risk of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Frontotemporal Degener. 22, 133–143 (2021).
Steyn, F. J. et al. Altered skeletal muscle glucose–fatty acid flux in amyotrophic lateral sclerosis. Brain Commun. 2, fcaa154 (2020).
Dupuis, L., Oudart, H., Rene, F., Gonzalez de Aguilar, J. L. & Loeffler, J. P. Evidence for defective energy homeostasis in amyotrophic lateral sclerosis: benefit of a high-energy diet in a transgenic mouse model. Proc. Natl Acad. Sci. USA 101, 11159–11164 (2004).
Boillee, S. et al. Onset and progression in inherited ALS determined by motor neurons and microglia. Science 312, 1389–1392 (2006).
Coughlan, K. S., Halang, L., Woods, I. & Prehn, J. H. A high-fat jelly diet restores bioenergetic balance and extends lifespan in the presence of motor dysfunction and lumbar spinal cord motor neuron loss in TDP-43A315T mutant C57BL6/J mice. Dis. Model. Mech. 9, 1029–1037 (2016).
Ludolph, A. C., Hugon, J., Dwivedi, M. P., Schaumburg, H. H. & Spencer, P. S. Studies on the aetiology and pathogenesis of motor neuron diseases. 1. Lathyrism: clinical findings in established cases. Brain 110, 149–165 (1987).
Hugon, J., Ludolph, A., Roy, D. N., Schaumburg, H. H. & Spencer, P. S. Studies on the etiology and pathogenesis of motor neuron diseases. II. Clinical and electrophysiologic features of pyramidal dysfunction in macaques fed Lathyrus sativus and IDPN. Neurology 38, 435–442 (1988).
Spencer, P. S. et al. Lathyrism: evidence for role of the neuroexcitatory aminoacid BOAA. Lancet 2, 1066–1067 (1986).
Zhang, L., Tang, L., Huang, T. & Fan, D. Life course adiposity and amyotrophic lateral sclerosis: a Mendelian randomization study. Ann. Neurol. 87, 434–441 (2020).
van Rheenen, W. et al. Author Correction: Common and rare variant association analyses in amyotrophic lateral sclerosis identify 15 risk loci with distinct genetic architectures and neuron-specific biology. Nat. Genet. 54, 361 (2022).
Bandres-Ciga, S. et al. Shared polygenic risk and causal inferences in amyotrophic lateral sclerosis. Ann. Neurol. 85, 470–481 (2019).
Esteban-Garcia, N. et al. Body complexion and circulating lipids in the risk of TDP-43 related disorders. Front. Aging Neurosci. 14, 838141 (2022).
Zeng, P., Wang, T., Zheng, J. & Zhou, X. Causal association of type 2 diabetes with amyotrophic lateral sclerosis: new evidence from Mendelian randomization using GWAS summary statistics. BMC Med. 17, 225 (2019).
Chen, H. et al. Type 2 diabetes mellitus and amyotrophic lateral sclerosis: genetic overlap, causality, and mediation. J. Clin. Endocrinol. Metab. 106, e4497–e4508 (2021).
Zhang, L., Tang, L., Huang, T. & Fan, D. Association between type 2 diabetes and amyotrophic lateral sclerosis. Sci. Rep. 12, 2544 (2022).
Hop, P. J. et al. Genome-wide study of DNA methylation shows alterations in metabolic, inflammatory, and cholesterol pathways in ALS. Sci. Transl. Med. 14, eabj0264 (2022).
Ngo, S. T., Mi, J. D., Henderson, R. D., McCombe, P. A. & Steyn, F. J. Exploring targets and therapies for amyotrophic lateral sclerosis: current insights into dietary interventions. Degener. Neurol. Neuro 7, 95–108 (2017).
Booth, F. W. Effect of limb immobilization on skeletal muscle. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 52, 1113–1118 (1982).
Burgos, R. et al. ESPEN guideline clinical nutrition in neurology. Clin. Nutr. 37, 354–396 (2018).
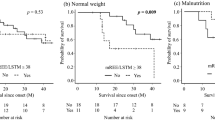
Lόpez-Gόmez, J. J. et al. Malnutrition at diagnosis in amyotrophic lateral sclerosis (als) and its influence on survival: using GLIM criteria. Clin. Nutr. 40, 237–244 (2021).
Gorges, M. et al. Hypothalamic atrophy is related to body mass index and age at onset in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 88, 1033–1041 (2017).
Chang, J. et al. Lower hypothalamic volume with lower body mass index is associated with shorter survival in patients with amyotrophic lateral sclerosis. Eur. J. Neurol. 30, 57–68 (2023).
Gabery, S. et al. Loss of the metabolism and sleep regulating neuronal populations expressing orexin and oxytocin in the hypothalamus in amyotrophic lateral sclerosis. Neuropathol. Appl. Neurobiol. 47, 979–989 (2021).
Desport, J. C. et al. Factors correlated with hypermetabolism in patients with amyotrophic lateral sclerosis. Am. J. Clin. Nutr. 74, 328–334 (2001).
Desport, J. C., Torny, F., Lacoste, M., Preux, P. M. & Couratier, P. Hypermetabolism in ALS: correlations with clinical and paraclinical parameters. Neurodegener. Dis. 2, 202–207 (2005).
Bouteloup, C. et al. Hypermetabolism in ALS patients: an early and persistent phenomenon. J. Neurol. 256, 1236–1242 (2009).
Funalot, B., Desport, J. C., Sturtz, F., Camu, W. & Couratier, P. High metabolic level in patients with familial amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 10, 113–117 (2009).
Vaisman, N. et al. Do patients with amyotrophic lateral sclerosis (ALS) have increased energy needs. J. Neurol. Sci. 279, 26–29 (2009).
Jésus, P. et al. Hypermetabolism is a deleterious prognostic factor in patients with amyotrophic lateral sclerosis. Eur. J. Neurol. 25, 97–104 (2018).
Steyn, F. J. et al. Hypermetabolism in ALS is associated with greater functional decline and shorter survival. J. Neurol. Neurosurg. Psychiatry 89, 1016–1023 (2018).
Jésus, P. et al. Increased resting energy expenditure compared with predictive theoretical equations in amyotrophic lateral sclerosis. Nutrition 77, 110805 (2020).
Ngo, S. T. et al. Progression and survival of patients with motor neuron disease relative to their fecal microbiota. Amyotroph. Lateral Scler. Frontotemporal Degener. 21, 549–562 (2020).
Fayemendy, P. et al. Hypermetabolism is a reality in amyotrophic lateral sclerosis compared to healthy subjects. J. Neurol. Sci. 420, 117257 (2021).
Nakamura, R. et al. Prognostic prediction by hypermetabolism varies depending on the nutritional status in early amyotrophic lateral sclerosis. Sci. Rep. 11, 17943 (2021).
Cattaneo, M. et al. The hypometabolic state: a good predictor of a better prognosis in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 93, 41–47 (2022).
He, J. et al. Hypermetabolism associated with worse prognosis of amyotrophic lateral sclerosis. J. Neurol. 269, 1447–1455 (2022).
Nakamura, R. et al. Investigation of the prognostic predictive value of serum lipid profiles in amyotrophic lateral sclerosis: roles of sex and hypermetabolism. Sci. Rep. 12, 1826 (2022).
Zurlo, F., Larson, K., Bogardus, C. & Ravussin, E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J. Clin. Invest. 86, 1423–1427 (1990).
Georges, M., Morelot-Panzini, C., Similowski, T. & Gonzalez-Bermejo, J. Noninvasive ventilation reduces energy expenditure in amyotrophic lateral sclerosis. BMC Pulm. Med. 14, 17 (2014).
Ferri, A. & Coccurello, R. What is “hyper” in the ALS hypermetabolism? Mediators Inflamm. 2017, 7821672 (2017).
Smith, E. F., Shaw, P. J. & De Vos, K. J. The role of mitochondria in amyotrophic lateral sclerosis. Neurosci. Lett. 710, 132933 (2019).
Dorst, J. et al. Metabolic alterations precede neurofilament changes in presymptomatic ALS gene carriers. EBioMedicine 90, 104521 (2023).
Genin, E. C. et al. CHCHD10 mutations promote loss of mitochondrial cristae junctions with impaired mitochondrial genome maintenance and inhibition of apoptosis. EMBO Mol. Med. 8, 58–72 (2016).
Genin, E. C. et al. Mitochondrial defect in muscle precedes neuromuscular junction degeneration and motor neuron death in CHCHD10S59L/+ mouse. Acta Neuropathol. 138, 123–145 (2019).
Genin, E. C. et al. Loss of MICOS complex integrity and mitochondrial damage, but not TDP-43 mitochondrial localisation, are likely associated with severity of CHCHD10-related diseases. Neurobiol. Dis. 119, 159–171 (2018).
Wang, T. et al. C9orf72 regulates energy homeostasis by stabilizing mitochondrial complex I assembly. Cell Metab. 33, 531–546.e9 (2021).
Onesto, E. et al. Gene-specific mitochondria dysfunctions in human TARDBP and C9ORF72 fibroblasts. Acta Neuropathol. Commun. 4, 47 (2016).
Gunther, R. et al. Alteration of mitochondrial integrity as upstream event in the pathophysiology of SOD1-ALS. Cells https://doi.org/10.3390/cells11071246 (2022).
Devoy, A. et al. Humanized mutant FUS drives progressive motor neuron degeneration without aggregation in ‘FUSDelta14’ knockin mice. Brain 140, 2797–2805 (2017).
Shan, X., Chiang, P. M., Price, D. L. & Wong, P. C. Altered distributions of Gemini of coiled bodies and mitochondria in motor neurons of TDP-43 transgenic mice. Proc. Natl Acad. Sci. USA 107, 16325–16330 (2010).
Izumikawa, K. et al. TDP-43 stabilises the processing intermediates of mitochondrial transcripts. Sci. Rep. 7, 7709 (2017).
Wang, W. et al. The inhibition of TDP-43 mitochondrial localization blocks its neuronal toxicity. Nat. Med. 22, 869–878 (2016).
Wang, P. et al. TDP-43 induces mitochondrial damage and activates the mitochondrial unfolded protein response. PLoS Genet. 15, e1007947 (2019).
Zuo, X. et al. TDP-43 aggregation induced by oxidative stress causes global mitochondrial imbalance in ALS. Nat. Struct. Mol. Biol. 28, 132–142 (2021).
Kawamata, H. et al. Mutant TDP-43 does not impair mitochondrial bioenergetics in vitro and in vivo. Mol. Neurodegener. 12, 37 (2017).
Dafinca, R. et al. Impairment of mitochondrial calcium buffering links mutations in C9ORF72 and TARDBP in iPS-derived motor neurons from patients with ALS/FTD. Stem Cell Rep. 14, 892–908 (2020).
Fazal, R. et al. HDAC6 inhibition restores TDP-43 pathology and axonal transport defects in human motor neurons with TARDBP mutations. EMBO J. 40, e106177 (2021).
Nakaya, T. & Maragkakis, M. Amyotrophic lateral sclerosis associated FUS mutation shortens mitochondria and induces neurotoxicity. Sci. Rep. 8, 15575 (2018).
Tsai, Y. L. et al. ALS/FTD-associated protein FUS induces mitochondrial dysfunction by preferentially sequestering respiratory chain complex mRNAs. Genes Dev. 34, 785–805 (2020).
Salam, S. et al. Identification of a novel interaction of FUS and syntaphilin may explain synaptic and mitochondrial abnormalities caused by ALS mutations. Sci. Rep. 11, 13613 (2021).
Stoica, R. et al. ALS/FTD-associated FUS activates GSK-3β to disrupt the VAPB-PTPIP51 interaction and ER-mitochondria associations. EMBO Rep. 17, 1326–1342 (2016).
Deng, J. et al. FUS interacts with HSP60 to promote mitochondrial damage. PLoS Genet. 11, e1005357 (2015).
Deng, J. et al. FUS interacts with ATP synthase beta subunit and induces mitochondrial unfolded protein response in cellular and animal models. Proc. Natl Acad. Sci. USA 115, E9678–E9686 (2018).
Briese, M. et al. Loss of Tdp-43 disrupts the axonal transcriptome of motoneurons accompanied by impaired axonal translation and mitochondria function. Acta Neuropathol. Commun. 8, 116 (2020).
Altman, T. et al. Axonal TDP-43 condensates drive neuromuscular junction disruption through inhibition of local synthesis of nuclear encoded mitochondrial proteins. Nat. Commun. 12, 6914 (2021).
Allen, S. P. et al. C9orf72 expansion within astrocytes reduces metabolic flexibility in amyotrophic lateral sclerosis. Brain 142, 3771–3790 (2019).
Allen, S. P. et al. Astrocyte adenosine deaminase loss increases motor neuron toxicity in amyotrophic lateral sclerosis. Brain 142, 586–605 (2019).
Yu, M. et al. Widespread mislocalization of FUS is associated with mitochondrial abnormalities in skeletal muscle in amyotrophic lateral sclerosis with FUS mutations. J. Neuropathol. Exp. Neurol. 81, 172–181 (2022).
Badu-Mensah, A., Guo, X., McAleer, C. W., Rumsey, J. W. & Hickman, J. J. Functional skeletal muscle model derived from SOD1-mutant ALS patient iPSCs recapitulates hallmarks of disease progression. Sci. Rep. 10, 14302 (2020).
Marini, C. et al. Mechanisms underlying the predictive power of high skeletal muscle uptake of FDG in amyotrophic lateral sclerosis. EJNMMI Res. 10, 76 (2020).
Dobrowolny, G. et al. Skeletal muscle is a primary target of SOD1G93A-mediated toxicity. Cell Metab. 8, 425–436 (2008).
Dobrowolny, G. et al. Metabolic changes associated with muscle expression of SOD1G93A. Front. Physiol. 9, 831 (2018).
Scaricamazza, S. et al. Skeletal-muscle metabolic reprogramming in ALS-SOD1G93A mice predates disease onset and is a promising therapeutic target. iScience 23, 101087 (2020).
Palamiuc, L. et al. A metabolic switch toward lipid use in glycolytic muscle is an early pathologic event in a mouse model of amyotrophic lateral sclerosis. EMBO Mol. Med. 7, 526–546 (2015).
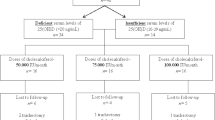
Wills, A. M. et al. Hypercaloric enteral nutrition in patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet 383, 2065–2072 (2014).
Dorst, J. et al. Percutaneous endoscopic gastrostomy in amyotrophic lateral sclerosis: a prospective observational study. J. Neurol. 262, 849–858 (2015).
Ludolph, A. C. et al. Effect of high-caloric nutrition on survival in amyotrophic lateral sclerosis. Ann. Neurol. 87, 206–216 (2020).
Dorst, J. et al. Effect of high-caloric nutrition on serum neurofilament light chain levels in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 91, 1007–1009 (2020).
Dorst, J. et al. Fat-rich versus carbohydrate-rich nutrition in ALS: a randomised controlled study. J. Neurol. Neurosurg. Psychiatry 93, 298–302 (2022).
Coates, E. et al. Patient, carer and healthcare professional perspectives on increasing calorie intake in amyotrophic lateral sclerosis. Chronic Illn. 19, 368–382 (2023).
Zarotti, N. et al. Health care professionals’ views on psychological factors affecting nutritional behaviour in people with motor neuron disease: a thematic analysis. Br. J. Health Psychol. 24, 953–969 (2019).
Biomed Central. ISCRTN registry https://www.isrctn.com/ISRCTN30588041 (2020).
Randle, P. J., Garland, P. B., Hales, C. N. & Newsholme, E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1, 785–789 (1963).
Ray, P. D., Huang, B. W. & Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 24, 981–990 (2012).
Browne, S. E. et al. Bioenergetic abnormalities in discrete cerebral motor pathways presage spinal cord pathology in the G93A SOD1 mouse model of ALS. Neurobiol. Dis. 22, 599–610 (2006).
Guo, Z., Kindy, M. S., Kruman, I. & Mattson, M. P. ALS-linked Cu/Zn-SOD mutation impairs cerebral synaptic glucose and glutamate transport and exacerbates ischemic brain injury. J. Cereb. Blood Flow. Metab. 20, 463–468 (2000).
Manzo, E. et al. Glycolysis upregulation is neuroprotective as a compensatory mechanism in ALS. Elife https://doi.org/10.7554/eLife.45114 (2019).
Martinez-Palma, L. et al. Mitochondrial modulation by dichloroacetate reduces toxicity of aberrant glial cells and gliosis in the SOD1G93A rat model of amyotrophic lateral sclerosis. Neurotherapeutics 16, 203–215 (2019).
Miyazaki, K. et al. Early and progressive impairment of spinal blood flow-glucose metabolism coupling in motor neuron degeneration of ALS model mice. J. Cereb. Blood Flow. Metab. 32, 456–467 (2012).
Weerasekera, A. et al. Non-invasive characterization of amyotrophic lateral sclerosis in a hTDP-43A315T mouse model: a PET-MR study. Neuroimage Clin. 27, 102327 (2020).
Desseille, C. et al. Specific physical exercise improves energetic metabolism in the skeletal muscle of amyotrophic-lateral-sclerosis mice. Front. Mol. Neurosci. 10, 332 (2017).
Smittkamp, S. E. et al. SOD1-G93A mice exhibit muscle-fiber-type-specific decreases in glucose uptake in the absence of whole-body changes in metabolism. Neurodegener. Dis. 13, 29–37 (2014).
Ferri, A. et al. Familial ALS-superoxide dismutases associate with mitochondria and shift their redox potentials. Proc. Natl Acad. Sci. USA 103, 13860–13865 (2006).
Jung, C., Higgins, C. M. & Xu, Z. Mitochondrial electron transport chain complex dysfunction in a transgenic mouse model for amyotrophic lateral sclerosis. J. Neurochem. 83, 535–545 (2002).
Kirkinezos, I. G. et al. Cytochrome c association with the inner mitochondrial membrane is impaired in the CNS of G93A-SOD1 mice. J. Neurosci. 25, 164–172 (2005).
Mattiazzi, M. et al. Mutated human SOD1 causes dysfunction of oxidative phosphorylation in mitochondria of transgenic mice. J. Biol. Chem. 277, 29626–29633 (2002).
Scaricamazza, S. et al. Repurposing of trimetazidine for amyotrophic lateral sclerosis: a study in SOD1G93A mice. Br. J. Pharmacol. 179, 1732–1752 (2022).
Allen, S. P. et al. Superoxide dismutase 1 mutation in a cellular model of amyotrophic lateral sclerosis shifts energy generation from oxidative phosphorylation to glycolysis. Neurobiol. Aging 35, 1499–1509 (2014).
Chaytow, H. et al. Targeting phosphoglycerate kinase 1 with terazosin improves motor neuron phenotypes in multiple models of amyotrophic lateral sclerosis. EBioMedicine 83, 104202 (2022).
Bannwarth, S. et al. A mitochondrial origin for frontotemporal dementia and amyotrophic lateral sclerosis through CHCHD10 involvement. Brain 137, 2329–2345 (2014).
Hor, J. H. et al. ALS motor neurons exhibit hallmark metabolic defects that are rescued by SIRT3 activation. Cell Death Differ. 28, 1379–1397 (2021).
Mehta, A. R. et al. Mitochondrial bioenergetic deficits in C9orf72 amyotrophic lateral sclerosis motor neurons cause dysfunctional axonal homeostasis. Acta Neuropathol. 141, 257–279 (2021).
Singh, T. et al. Neuronal mitochondrial dysfunction in sporadic amyotrophic lateral sclerosis is developmentally regulated. Sci. Rep. 11, 18916 (2021).
Crugnola, V. et al. Mitochondrial respiratory chain dysfunction in muscle from patients with amyotrophic lateral sclerosis. Arch. Neurol. 67, 849–854 (2010).
Dodge, J. C. et al. Metabolic signatures of amyotrophic lateral sclerosis reveal insights into disease pathogenesis. Proc. Natl Acad. Sci. USA 110, 10812–10817 (2013).
Echaniz-Laguna, A. et al. Muscular mitochondrial function in amyotrophic lateral sclerosis is progressively altered as the disease develops: a temporal study in man. Exp. Neurol. 198, 25–30 (2006).
Echaniz-Laguna, A. et al. Mitochondrial respiratory chain function in skeletal muscle of ALS patients. Ann. Neurol. 52, 623–627 (2002).
Canosa, A. et al. Brain metabolic changes across King’s stages in amyotrophic lateral sclerosis: a 18F-2-fluoro-2-deoxy-d-glucose-positron emission tomography study. Eur. J. Nucl. Med. Mol. Imaging 48, 1124–1133 (2021).
Canosa, A. et al. 18F-FDG-PET correlates of cognitive impairment in ALS. Neurology 86, 44–49 (2016).
Cistaro, A. et al. The metabolic signature of C9ORF72-related ALS: FDG PET comparison with nonmutated patients. Eur. J. Nucl. Med. Mol. Imaging 41, 844–852 (2014).
Cistaro, A. et al. Brain hypermetabolism in amyotrophic lateral sclerosis: a FDG PET study in ALS of spinal and bulbar onset. Eur. J. Nucl. Med. Mol. Imaging 39, 251–259 (2012).
Dalakas, M. C., Hatazawa, J., Brooks, R. A. & Di Chiro, G. Lowered cerebral glucose utilization in amyotrophic lateral sclerosis. Ann. Neurol. 22, 580–586 (1987).
Hatazawa, J., Brooks, R. A., Dalakas, M. C., Mansi, L. & Di Chiro, G. Cortical motor-sensory hypometabolism in amyotrophic lateral sclerosis: a PET study. J. Comput. Assist. Tomogr. 12, 630–636 (1988).
Ludolph, A. C. et al. Frontal lobe function in amyotrophic lateral sclerosis: a neuropsychologic and positron emission tomography study. Acta Neurol. Scand. 85, 81–89 (1992).
Marini, C. et al. Interplay between spinal cord and cerebral cortex metabolism in amyotrophic lateral sclerosis. Brain 141, 2272–2279 (2018).
Pagani, M. et al. Functional pattern of brain FDG-PET in amyotrophic lateral sclerosis. Neurology 83, 1067–1074 (2014).
Yamashita, T. et al. Flow-metabolism uncoupling in the cervical spinal cord of ALS patients. Neurol. Sci. 38, 659–665 (2017).
Andreassen, O. A. et al. Increases in cortical glutamate concentrations in transgenic amyotrophic lateral sclerosis mice are attenuated by creatine supplementation. J. Neurochem. 77, 383–390 (2001).
Klivenyi, P., Gardian, G., Calingasan, N. Y., Yang, L. & Beal, M. F. Additive neuroprotective effects of creatine and a cyclooxygenase 2 inhibitor against dopamine depletion in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson’s disease. J. Mol. Neurosci. 21, 191–198 (2003).
Bordet, T. et al. Identification and characterization of cholest-4-en-3-one, oxime (TRO19622), a novel drug candidate for amyotrophic lateral sclerosis. J. Pharmacol. Exp. Ther. 322, 709–720 (2007).
Thams, S. et al. A stem cell-based screening platform identifies compounds that desensitize motor neurons to endoplasmic reticulum stress. Mol. Ther. 27, 87–101 (2019).
Schutz, B. et al. The oral antidiabetic pioglitazone protects from neurodegeneration and amyotrophic lateral sclerosis-like symptoms in superoxide dismutase-G93A transgenic mice. J. Neurosci. 25, 7805–7812 (2005).
Elia, A. E. et al. Tauroursodeoxycholic acid in the treatment of patients with amyotrophic lateral sclerosis. Eur. J. Neurol. 23, 45–52 (2016).
Dupuis, L. et al. A randomized, double blind, placebo-controlled trial of pioglitazone in combination with riluzole in amyotrophic lateral sclerosis. PLoS ONE 7, e37885 (2012).
Vercruysse, P. et al. Alterations in the hypothalamic melanocortin pathway in amyotrophic lateral sclerosis. Brain 139, 1106–1122 (2016).
Luu, L. C. T., Kasarskis, E. J. & Tandan, R. in Amyotrophic Lateral Sclerosis Ch. 32 (eds Mitsumoto, H, Przedborski, S. & Gordon, P. H.) 721–735 (Taylor & Francis, 2006).
Robison, R. et al. Swallowing safety and efficiency impairment profiles in individuals with amyotrophic lateral sclerosis. Dysphagia 37, 644–654 (2022).
Belafsky, P. C. et al. Validity and reliability of the Eating Assessment Tool (EAT-10). Ann. Otol. Rhinol. Laryngol. 117, 919–924 (2008).
Cedarbaum, J. M. et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J. Neurol. Sci. 169, 13–21 (1999).
Strand, E. A., Miller, R. M., Yorkston, K. M. & Hillel, A. D. Management of oral-pharyngeal dysphagia symptoms in amyotrophic lateral sclerosis. Dysphagia 11, 129–139 (1996).
Shim, J. S., Oh, K. & Kim, H. C. Dietary assessment methods in epidemiologic studies. Epidemiol. Health 36, e2014009 (2014).
Trabulsi, J. & Schoeller, D. A. Evaluation of dietary assessment instruments against doubly labeled water, a biomarker of habitual energy intake. Am. J. Physiol. Endocrinol. Metab. 281, E891–E899 (2001).
Kasarskis, E. J. et al. Estimating daily energy expenditure in individuals with amyotrophic lateral sclerosis. Am. J. Clin. Nutr. 99, 792–803 (2014).
Shimizu, T. et al. The measurement and estimation of total energy expenditure in Japanese patients with ALS: a doubly labelled water method study. Amyotroph. Lateral Scler. Frontotemporal Degener. 18, 37–45 (2017).
Lopes da Silva, H. F. et al. Dietary intake and zinc status in amyotrophic lateral sclerosis patients. Nutr. Hosp. 34, 1361–1367 (2017).
Barros, A., Dourado, M. E. T. Jr., Pedrosa, L. F. C. & Leite-Lais, L. Association of copper status with lipid profile and functional status in patients with amyotrophic lateral sclerosis. J. Nutr. Metab. 2018, 5678698 (2018).
Chelstowska, B. & Kuzma-Kozakiewicz, M. Biochemical parameters in determination of nutritional status in amyotrophic lateral sclerosis. Neurol. Sci. 41, 1115–1124 (2020).
Lemos, T. & Gallagher, D. Current body composition measurement techniques. Curr. Opin. Endocrinol. Diabetes Obes. 24, 310–314 (2017).
Keys, A., Brozek, J., Henschel, A., Mickelson, O. & Taylor, H. L. The Biology of Human Starvation (University of Minnesota Press, 1950).
Park, J.-W. et al. Body fat percentage and availability of oral food intake: prognostic factors and implications for nutrition in amyotrophic lateral sclerosis. Nutrients 13, 3704 (2021).
University of Kentucky College of Medicine. ALS Nutrition Calculator. University of Kentucky College of Medicine https://alsnutrcalc.ukhc.org/calc (2023).
Sherman, M. S., Pillai, A., Jackson, A. & Heiman-Patterson, T. Standard equations are not accurate in assessing resting energy expenditure in patients with amyotrophic lateral sclerosis. J. Parenter. Enter. Nutr. 28, 442–446 (2004).
Pontzer, H. et al. Daily energy expenditure through the human life course. Science 373, 808–812 (2021).
Acknowledgements
C.McD. is supported by the NIHR Sheffield Biomedical Research Centre and an NIHR Research Professorship Award. S.N. is supported by a FightMND Mid-Career Research Fellowship.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neurology thanks Andrea Calvo, Philippe Corcia and Dongsheng Fan for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ludolph, A., Dupuis, L., Kasarskis, E. et al. Nutritional and metabolic factors in amyotrophic lateral sclerosis. Nat Rev Neurol 19, 511–524 (2023). https://doi.org/10.1038/s41582-023-00845-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-023-00845-8
This article is cited by
-
Genome-wide DNA methylation analysis related to ALS patient progression and survival
Journal of Neurology (2024)