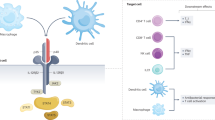
Abstract
Cytokines produced by immune cells contribute to the development and perpetuation of inflammatory bowel disease (IBD), namely Crohn’s disease and ulcerative colitis, by regulating various aspects of the inflammatory response. Pro-inflammatory cytokines trigger chronic intestinal inflammation, tissue damage, carcinogenesis and perpetuation of disease and suppress the resolution of inflammation in IBD. The clinical success of antibodies that neutralize tumour necrosis factor (TNF) and the cytokine IL-12p40 in individuals with IBD has underscored this concept. Moreover, genetic and preclinical studies have emphasized the crucial role of IL-23 in IBD, leading to clinical approval of antibodies targeting this cytokine. Multiple studies have also investigated the administration of cytokines with assumed anti-inflammatory effects, but this approach has yet to show any real clinical benefit in individuals with IBD. Recent studies have targeted the cytokine network through the use of multi-cytokine blockers (for example, Janus kinase (JAK) inhibitors), IL-2-induced regulatory T cells or advanced combination therapies that use multiple cytokine blockers simultaneously (for example, anti-TNF along with anti-IL-23 antibodies). This Review will focus on our current understanding of how cytokines produced by innate and adaptive immune cells contribute to IBD pathogenesis and discuss how their modulation may inform future treatments for IBD.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Altan-Bonnet, G. & Mukherjee, R. Cytokine-mediated communication: a quantitative appraisal of immune complexity. Nat. Rev. Immunol. 19, 205–217 (2019).
Neurath, M. F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 14, 329–342 (2014).
Fan, H. et al. Innate lymphoid cells: regulators of gut barrier function and immune homeostasis. J. Immunol. Res. 2019, 2525984 (2019).
Fuchs, A. et al. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-γ-producing cells. Immunity 38, 769–781 (2013).
Bernink, J. H., Germar, K. & Spits, H. The role of ILC2 in pathology of type 2 inflammatory diseases. Curr. Opin. Immunol. 31, 115–120 (2014).
Rao, A. et al. Cytokines regulate the antigen-presenting characteristics of human circulating and tissue-resident intestinal ILCs. Nat. Commun. 11, 2049 (2020).
Coombes, J. L. et al. A functionally specialized population of mucosal CD103 DCs induces Foxp3 regulatory T cells via a TGF-β- and retinoic acid-dependent mechanism. J. Exp. Med. 204, 1757–1764 (2007).
Nakahashi-Oda, C. et al. Apoptotic epithelial cells control the abundance of Treg cells at barrier surfaces. Nat. Immunol. 17, 441–450 (2016).
Olszak, T. et al. Protective mucosal immunity mediated by epithelial CD1d and IL-10. Nature 509, 497–502 (2014). This work is a key paper on the role of IL-10 and epithelial cell function.
Lee, C. H., Koh, S. J., Radi, Z. A. & Habtezion, A. Animal models of inflammatory bowel disease: novel experiments for revealing pathogenesis of colitis, fibrosis, and colitis-associated colon cancer. Intest. Res. 21, 295–305 (2023).
Jans, D. & Cleynen, I. The genetics of non-monogenic IBD. Hum. Genet. 142, 669–682 (2023).
Al-Sadi, R., Ye, D., Dokladny, K. & Ma, T. Y. Mechanism of IL-1β-induced increase in intestinal epithelial tight junction permeability. J. Immunol. 180, 5653–5661 (2008).
Shouval, D. S. et al. Interleukin 1β mediates intestinal inflammation in mice and patients with interleukin 10 receptor deficiency. Gastroenterology 151, 1100–1104 (2016).
Wang, Y. et al. Neutrophil infiltration favors colitis-associated tumorigenesis by activating the interleukin-1 (IL-1)/IL-6 axis. Mucosal Immunol. 7, 1106–1115 (2014).
Coccia, M. et al. IL-1β mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4+ TH17 cells. J. Exp. Med. 209, 1595–1609 (2012).
Aschenbrenner, D. et al. Deconvolution of monocyte responses in inflammatory bowel disease reveals an IL-1 cytokine network that regulates IL-23 in genetic and acquired IL-10 resistance. Gut 70, 1023–1036 (2021).
Kaminsky, L. W., Al-Sadi, R. & Ma, T. Y. IL-1β and the intestinal epithelial tight junction barrier. Front. Immunol. 12, 767456 (2021).
Friedrich, M. et al. IL-1-driven stromal–neutrophil interactions define a subset of patients with inflammatory bowel disease that does not respond to therapies. Nat. Med. 27, 1970–1981 (2021).
de Luca, A. et al. IL-1 receptor blockade restores autophagy and reduces inflammation in chronic granulomatous disease in mice and in humans. Proc. Natl Acad. Sci. USA 111, 3526–3531 (2014).
Liso, M. et al. Interleukin 1β blockade reduces intestinal inflammation in a murine model of tumor necrosis factor-independent ulcerative colitis. Cell Mol. Gastroenterol. Hepatol. 14, 151–171 (2022).
Raine, T. et al. Results of a randomised controlled trial to evaluate interleukin 1 blockade with anakinra in patients with acute severe ulcerative colitis (IASO). J. Crohn’s Colitis 17, i43–i46 (2023).
Wirtz, S., Becker, C., Blumberg, R., Galle, P. R. & Neurath, M. F. Treatment of T cell-dependent experimental colitis in SCID mice by local administration of an adenovirus expressing IL-18 antisense mRNA. J. Immunol. 168, 411–420 (2002).
Nowarski, R. et al. Epithelial IL-18 equilibrium controls barrier function in colitis. Cell 163, 1444–1456 (2015).
Kanai, T. et al. Macrophage-derived IL-18-mediated intestinal inflammation in the murine model of Crohn’s disease. Gastroenterology 121, 875–888 (2001).
Gao, H. et al. Dysregulated microbiota-driven gasdermin d activation promotes colitis development by mediating IL-18 release. Front. Immunol. 12, 750841 (2021).
Siegmund, B., Lehr, H. A., Fantuzzi, G. & Dinarello, C. A. IL-1β-converting enzyme (caspase-1) in intestinal inflammation. Proc. Natl Acad. Sci. USA 98, 13249–13254 (2001).
Bauer, C. et al. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 59, 1192–1199 (2010).
Castro-Dopico, T. et al. Anti-commensal IgG drives intestinal inflammation and type 17 immunity in ulcerative colitis. Immunity 50, 1099–1114.e10 (2019).
Toskas, A. et al. Expression of IL-21 and IL-33 in intestinal mucosa of inflammatory bowel disease: an immunohistochemical study. Diagnostics 13, 2185 (2023).
Pastorelli, L. et al. Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental TH1/TH2 driven enteritis. Proc. Natl Acad. Sci. USA 107, 8017–8022 (2010).
Waddell, A., Vallance, J. E., Fox, S. & Rosen, M. J. IL-33 is produced by colon fibroblasts and differentially regulated in acute and chronic murine colitis. Sci. Rep. 11, 9575 (2021).
Oboki, K. et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc. Natl Acad. Sci. USA 107, 18581–18586 (2010).
Schiering, C. et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 513, 564–568 (2014). This paper uncovers a novel role of IL-33 in controlling intestinal Treg cell function.
Zhu, S., Zhang, J., Jiang, X., Wang, W. & Chen, Y. Q. Free fatty acid receptor 4 deletion attenuates colitis by modulating Treg cells via ZBED6–IL33 pathway. eBioMedicine 80, 104060 (2022).
He, Z. et al. Mast cells are essential intermediaries in regulating IL-33/ST2 signaling for an immune network favorable to mucosal healing in experimentally inflamed colons. Cell Death Dis. 9, 1173 (2018).
Russell, S. E. et al. IL-36ɑ expression is elevated in ulcerative colitis and promotes colonic inflammation. Mucosal Immunol. 9, 1193–1204 (2016).
Scheibe, K. et al. IL-36R signalling activates intestinal epithelial cells and fibroblasts and promotes mucosal healing in vivo. Gut 66, 823–838 (2017).
Medina-Contreras, O. et al. Cutting edge: IL-36 receptor promotes resolution of intestinal damage. J. Immunol. 196, 34–38 (2016).
Ngo, V. L. et al. A cytokine network involving IL-36γ, IL-23, and IL-22 promotes antimicrobial defense and recovery from intestinal barrier damage. Proc. Natl Acad. Sci. USA 115, E5076–E5085 (2018).
Scheibe, K. et al. Inhibiting interleukin 36 receptor signaling reduces fibrosis in mice with chronic intestinal inflammation. Gastroenterology 156, 1082–1097.e11 (2019).
Elias, M. et al. IL-36 in chronic inflammation and fibrosis — bridging the gap? J. Clin. Invest. 131, e144336 (2021).
Leon, G. et al. IL-36 cytokines imprint a colitogenic phenotype on CD4+ T helper cells. Mucosal Immunol. 15, 491–503 (2022).
Ferrante, M. et al. Safety and tolerability of spesolimab in patients with ulcerative colitis. Expert. Opin. Drug. Saf. 22, 141–152 (2023).
Cavalli, G. & Dinarello, C. A. Suppression of inflammation and acquired immunity by IL-37. Immunol. Rev. 281, 179–190 (2018).
Imaeda, H. et al. Epithelial expression of interleukin-37b in inflammatory bowel disease. Clin. Exp. Immunol. 172, 410–416 (2013).
McNamee, E. N. et al. Interleukin 37 expression protects mice from colitis. Proc. Natl Acad. Sci. USA 108, 16711–16716 (2011).
Krohn, L. et al. Modulation of intestinal IL-37 expression and its impact on the epithelial innate immune response and barrier integrity. Front. Immunol. 14, 1261666 (2023).
Mountford, S. et al. Interleukin-37 inhibits colon carcinogensis during chronic colitis. Front. Immunol. 10, 2632 (2019).
Wang, W. Q. et al. IL-37b gene transfer enhances the therapeutic efficacy of mesenchumal stromal cells in DSS-induced colitis mice. Acta Pharmacol. Sin. 36, 1377–1387 (2015).
Cong, J. et al. Interleukin-37 exacerbates experimental colitis in an intestinal microbiome-dependent fashion. Theranostics 12, 5204–5219 (2022).
Boutet, M. A. et al. Distinct expression of interleukin (IL)-36ɑ, β and γ, their antagonist IL-36Ra and IL-38 in psoriasis, rheumatoid arthritis and Crohn’s disease. Clin. Exp. Immunol. 184, 159–173 (2016).
Xie, C. et al. Interleukin-38 is elevated in inflammatory bowel diseases and suppresses intestinal inflammation. Cytokine 127, 154963 (2020).
Ohno, M. et al. The anti-inflammatory and protective role of interleukin-38 in inflammatory bowel disease. J. Clin. Biochem. Nutr. 70, 64–71 (2022).
de Graaf, D. M. et al. IL-38 gene deletion worsens murine colitis. Front. Immunol. 13, 840719 (2022).
Rose-John, S., Jenkins, B. J., Garbers, C., Moll, J. M. & Scheller, J. Targeting IL-6 trans-signalling: past, present and future prospects. Nat. Rev. Immunol. 23, 666–681 (2023).
Atreya, R. et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn disease and experimental colitis in vivo. Nat. Med. 6, 583–588 (2000). This work is the first manuscript to describe the role of sIL-6R signalling in IBD.
Danese, S. et al. Randomised trial and open-label extension study of an anti-interleukin-6 antibody in Crohn’s disease (ANDANTE I and II). Gut 68, 40–48 (2019).
Schreiber, S. et al. Therapeutic interleukin-6 trans-signaling inhibition by olamkicept (sgp130Fc) in patients with active inflammatory bowel disease. Gastroenterology 160, 2354–2366.e11 (2021). This paper presents an early clinical trial on the blockade of IL-6 trans-signalling in IBD.
PR Newswire. I-Mab announces positive topline phase 2 results for olamkicept in ulcerative colitis. PR Newswire https://www.prnewswire.com/news-releases/i-mab-announces-positive-topline-phase-2-results-for-olamkicept-in-ulcerative-colitis-301276622.html (2021).
Becker, C. et al. TGF-β suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity 21, 491–501 (2004).
Grivennikov, S. et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 15, 103–113 (2009).
Nishina, T. et al. Interleukin 11 confers resistance to dextran sulfate sodium-induced colitis in mice. iScience 26, 105934 (2023).
Gibson, D. L. et al. Interleukin-11 reduces TLR4-induced colitis in TLR2-deficient mice and restores intestinal STAT3 signaling. Gastroenterology 139, 1277–1288 (2010).
Herrlinger, K. R. et al. Randomized, double blind controlled trial of subcutaneous recombinant human interleukin-11 versus prednisolone in active Crohn’s disease. Am. J. Gastroenterol. 101, 793–797 (2006).
Guimbaud, R. et al. Leukemia inhibitory factor involvement in human ulcerative colitis and its potential role in malignant course. Eur. Cytokine Netw. 9, 607–612 (1998).
Zhang, Y. S. et al. STAT4 activation by leukemia inhibitory factor confers a therapeutic effect on intestinal inflammation. EMBO J. 38, e99595 (2019).
West, N. R. et al. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nat. Med. 23, 579–589 (2017). This work is a key paper on the role of OSM in intestinal inflammation in patients failing anti-TNF therapy.
Verstockt, S. et al. Oncostatin M is a biomarker of diagnosis, worse disease prognosis, and therapeutic nonresponse in inflammatory bowel disease. Inflamm. Bowel Dis. 27, 1564–1575 (2021).
Guo, A. et al. High oncostatin M predicts lack of clinical remission for patients with inflammatory bowel disease on tumor necrosis factor ɑ antagonists. Sci. Rep. 12, 1185 (2022).
van Loo, G. & Bertrand, M. J. M. Death by TNF: a road to inflammation. Nat. Rev. Immunol. 23, 289–303 (2023).
Atreya, R. et al. Antibodies against tumor necrosis factor (TNF) induce T-cell apoptosis in patients with inflammatory bowel diseases via TNF receptor 2 and intestinal CD14+ macrophages. Gastroenterology 141, 2026–2038 (2011).
Corazza, N. et al. Transmembrane tumor necrosis factor is a potent inducer of colitis even in the absence of its secreted form. Gastroenterology 127, 816–825 (2004).
Hess, A. et al. Functional brain imaging reveals rapid blockade of abdominal pain response upon anti-TNF therapy in Crohn’s disease. Gastroenterology 149, 864–866 (2015).
Cozzi, G. et al. Spondyloarthritis with inflammatory bowel disease: the latest on biologic and targeted therapies. Nat. Rev. Rheumatol. 19, 503–518 (2023).
Perrier, C. et al. Neutralization of membrane TNF, but not soluble TNF, is crucial for the treatment of experimental colitis. Inflamm. Bowel Dis. 19, 246–253 (2013).
Sandborn, W. J. et al. Etanercept for active Crohn’s disease: a randomized, double-blind, placebo-controlled trial. Gastroenterology 121, 1088–1094 (2001).
Atreya, R. et al. In vivo molecular imaging using fluorescent anti-TNF antibodies predicts response to biological therapy in Crohn’s disease. Nat. Med. 52, 313–318 (2014).
Kokkotis, G. & Bamias, G. TL1A as a therapeutic target in inflammatory bowel disease. Expert. Rev. Clin. Immunol. 18, 551–555 (2022).
Jacob, N. et al. Direct signaling of TL1A–DR3 on fibroblasts induces intestinal fibrosis in vivo. Sci. Rep. 10, 18189 (2020).
Bamias, G. et al. Expression, localization, and functional activity of TL1A, a novel TH1-polarizing cytokine in inflammatory bowel disease. J. Immunol. 171, 4868–4874 (2003).
Castellanos, J. G. et al. Microbiota-induced TNF-like ligand 1A drives group 3 innate lymphoid cell-mediated barrier protection and intestinal T cell activation during colitis. Immunity 49, 1077–1089 e1075 (2018).
Jin, S. et al. TL1A/TNFSF15 directly induces proinflammatory cytokines, including TNFɑ, from CD3+CD161+ T cells to exacerbate gut inflammation. Mucosal Immunol. 6, 886–899 (2013).
Pai, Y. C. et al. Gut microbial transcytosis induced by tumor necrosis factor-like 1A-dependent activation of a myosin light chain kinase splice variant contributes to IBD. J. Crohn’s Colitis 15, 258–272 (2020).
Sands, B. et al. PRA023 demonstrated efficacy and favorable safety as induction therapy for moderately to severely active UC: phase 2 ARTEMIS-UC study results. European Crohn’s and Colitis Organisation https://www.ecco-ibd.eu/publications/congress-abstracts/item/op40-pra023-demonstrated-efficacy-and-favorable-safety-as-induction-therapy-for-moderately-to-severely-active-uc-phase-2-artemis-uc-study-results.html (2023).
Egea, L. et al. GM-CSF produced by nonhematopoietic cells is required for early epithelial cell proliferation and repair of injured colonic mucosa. J. Immunol. 190, 1702–1713 (2013).
Hu, Y. et al. Interleukin-1β-induced IRAK1 ubiquitination is required for TH–GM-CSF cell differentiation in T cell-mediated inflammation. J. Autoimmun. 102, 50–64 (2019).
Pearson, C. et al. ILC3 GM-CSF production and mobilisation orchestrate acute intestinal inflammation. eLife 5, e10066 (2016).
Song, C. et al. Unique and redundant functions of NKp46+ ILC3s in models of intestinal inflammation. J. Exp. Med. 212, 1869–1882 (2015).
Ariki, S. et al. GM-CSF-producing CCR2+CCR6+ TH17 cells are pathogenic in dextran sodium sulfate-induced colitis model in mice. Genes Cell 28, 267–276 (2023).
Griseri, T. et al. Granulocyte macrophage colony-stimulating factor-activated eosinophils promote interleukin-23 driven chronic colitis. Immunity 43, 187–199 (2015).
Bernasconi, E. et al. Granulocyte–macrophage colony-stimulating factor elicits bone marrow-derived cells that promote efficient colonic mucosal healing. Inflamm. Bowel Dis. 16, 428–441 (2010).
Sainathan, S. K. et al. Granulocyte macrophage colony-stimulating factor ameliorates DSS-induced experimental colitis. Inflamm. Bowel Dis. 14, 88–99 (2008).
Xu, Y., Hunt, N. H. & Bao, S. The role of granulocyte macrophage-colony-stimulating factor in acute intestinal inflammation. Cell Res. 18, 1220–1229 (2008).
Gilbert, S. et al. Enterocyte STAT5 promotes mucosal wound healing via suppression of myosin light chain kinase-mediated loss of barrier function and inflammation. EMBO Mol. Med. 4, 109–124 (2012).
Wang, Y. et al. Tumor-derived GM-CSF promotes inflammatory colon carcinogenesis via stimulating epithelial release of VEGF. Cancer Res. 74, 716–726 (2014).
Mortha, A. et al. Neutralizing anti-granulocyte macrophage-colony stimulating factor autoantibodies recognize post-translational glycosylations on granulocyte macrophage-colony stimulating factor years before diagnosis and predict complicated Crohn’s disease. Gastroenterology 163, 659–670 (2022).
Chuang, L. S. et al. A frameshift in CSF2RB predominant among Ashkenazi Jews increases risk for Crohn’s disease and reduces monocyte signaling via GM-CSF. Gastroenterology 151, 710–723.2 (2016).
Agnholt, J., Kelsen, J., Brandsborg, B., Jakobsen, N. O. & Dahlerup, J. F. Increased production of granulocyte–macrophage colony-stimulating factor in Crohn’s disease — a possible target for infliximab treatment. Eur. J. Gastroenterol. Hepatol. 16, 649–655 (2004).
Goldstein, J. I. et al. Defective leukocyte GM-CSF receptor (CD116) expression and function in inflammatory bowel disease. Gastroenterology 141, 208–216 (2011).
Roth, L., Macdonald, J. K., McDonald, J. W. & Chande, N. Sargramostim (GM-CSF) for induction of remission in Crohn’s disease. Cochrane Database Syst. Rev. 9, CD008538 (2011).
Ullrich, K. A. et al. IL-3 receptor signalling suppresses chronic intestinal inflammation by controlling mechanobiology and tissue egress of regulatory T cells. Gut 72, 2081–2094 (2023).
Benard, A. et al. IL-3 orchestrates ulcerative colitis pathogenesis by controlling the development and the recruitment of splenic reservoir neutrophils. Cell Rep. 42, 112637 (2023).
Abo, H. et al. Combined IL-2 immunocomplex and anti-IL-5 mAb treatment expands Foxp3+ Treg cells in the absence of eosinophilia and ameliorates experimental colitis. Front. Immunol. 10, 459 (2019).
Stevceva, L. et al. Eosinophilia is attenuated in experimental colitis induced in IL-5 deficient mice. Genes. Immun. 1, 213–218 (2000).
Yamada, A. et al. Role of regulatory T cell in the pathogenesis of inflammatory bowel disease. World J. Gastroenterol. 22, 2195–2205 (2016).
Bauche, D. et al. IL-23 and IL-2 activation of STAT5 is required for optimal IL-22 production in ILC3s during colitis. Sci. Immunol. 5, eaav1080 (2020).
Tchitchek, N. et al. Low-dose IL-2 shapes a tolerogenic gut microbiota that improves autoimmunity and gut inflammation. JCI insight 7, e159406 (2022).
Allegretti, J. R., Mitsialis, V., Canavan, J. B., Low-Dose IL2 UC Study Group & Snapper, S. B. Low-dose interleukin 2 for the treatment of moderate to severe ulcerative colitis. Gastroenterology 165, 492–495.e2 (2023).
Dardalhon, V. et al. IL-4 inhibits TGF-β-induced Foxp3+ T cells and, together with TGF-β, generates IL-9+IL-10+Foxp3– effector T cells. Nat. Immunol. 9, 1347–1355 (2008).
Specht, S., Arriens, S. & Hoerauf, A. Induction of chronic colitis in IL-10 deficient mice requires IL-4. Microbes Infect. 8, 694–703 (2006).
Belarif, L. et al. IL-7 receptor influences anti-TNF responsiveness and T cell gut homing in inflammatory bowel disease. J. Clin. Invest. 129, 1910–1925 (2019).
Shinohara, T. et al. Upregulated IL-7 receptor ɑ expression on colitogenic memory CD4+ T cells may participate in the development and persistence of chronic colitis. J. Immunol. 186, 2623–2632 (2011).
Ji, T. et al. Aryl hydrocarbon receptor activation down-regulates IL-7 and reduces inflammation in a mouse model of DSS-induced colitis. Dig. Dis. Sci. 60, 1958–1966 (2015).
Nalleweg, N. et al. IL-9 and its receptor are predominantly involved in the pathogenesis of UC. Gut 64, 743–755 (2014).
Gerlach, K. et al. TH9 cells that express the transcription factor PU.1 drive T cell-mediated colitis via IL-9 receptor signaling in intestinal epithelial cells. Nat. Immunol. 15, 676–686 (2014).
de Heusch, M. et al. IL-9 exerts biological function on antigen-experienced murine T cells and exacerbates colitis induced by adoptive transfer. Eur. J. Immunol. 50, 1034–1043 (2020).
Bamidele, A. O. et al. Interleukin-21 drives a hypermetabolic state and CD4+ T cell-associated pathogenicity in chronic intestinal inflammation. Gastroenterologyhttps://doi.org/10.1053/j.gastro.2024.01.026 (2024).
Fina, D. et al. Regulation of gut inflammation and TH17 cell response by interleukin-21. Gastroenterology 134, 1038–1048 (2008).
Stolfi, C. et al. Involvement of interleukin-21 in the regulation of colitis-associated colon cancer. J. Exp. Med. 208, 2279–2290 (2011).
Monteleone, G. et al. Interleukin 12 is expressed and actively released by Crohn’s disease intestinal lamina propria mononuclear cells. Gastroenterology 112, 1169–1178 (1997).
Uhlig, H. H. et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity 25, 309–318 (2006).
Eftychi, C. et al. Temporally distinct functions of the cytokines IL-12 and IL-23 drive chronic colon inflammation in response to intestinal barrier impairment. Immunity 51, 367–380.e4 (2019).
Neurath, M. F. IL-23 in inflammatory bowel diseases and colon cancer. Cytokine Growth Factor. Rev. 45, 1–8 (2019).
D’Haens, G. et al. Risankizumab as induction therapy for Crohn’s disease: results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet 399, 2015–2030 (2022). This work presents a large clinical phase III study on the use of an anti-IL-23 antibody in IBD.
Sands, B. E. et al. Efficacy and safety of mirikizumab in a randomized phase 2 study of patients with Crohn’s disease. Gastroenterology 162, 495–508 (2022).
Sandborn, W. J. et al. Guselkumab for the treatment of Crohn’s disease: induction results from the phase 2 GALAXI-1 study. Gastroenterology 162, 1650–1664.e8 (2022).
Hueber, W. et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 61, 1693–1700 (2012). This work presents the first clinical trial on the use of an anti-IL-17A blocker in IBD.
Maxwell, J. R. et al. Differential roles for interleukin-23 and interleukin-17 in intestinal immunoregulation. Immunity 43, 739–750 (2015).
Furuzawa Carballeda, J., Fonseca Camarillo, G. & Yamamoto-Furusho, J. K. Interleukin 27 is up-regulated in patients with active inflammatory bowel disease. Immunologic Res. 64, 901–907 (2016).
Villarino, A. V. et al. IL-27R deficiency delays the onset of colitis and protects from helminth-induced pathology in a model of chronic IBD. Int. Immunol. 20, 739–752 (2008).
Visperas, A., Do, J. S., Bulek, K., Li, X. & Min, B. IL-27, targeting antigen-presenting cells, promotes TH17 differentiation and colitis in mice. Mucosal Immunol. 7, 625–633 (2013).
Cox, J. H. et al. IL-27 promotes T cell-dependent colitis through multiple mechanisms. J. Exp. Med. 208, 115–123 (2011).
Troy, A. E. et al. IL-27 regulates homeostasis of the intestinal CD4+ effector T cell pool and limits intestinal inflammation in a murine model of colitis. J. Immunol. 183, 2037–2044 (2009).
Hanson, M. L. et al. Oral delivery of IL-27 recombinant bacteria attenuates immune colitis in mice. Gastroenterology 146, 210–221.e13 (2014).
Sasaoka, T. et al. Treatment with IL-27 attenuates experimental colitis through the suppression of the development of IL-17-producing T helper cells. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G568–G576 (2011).
Nie, M., Huang, D., Chen, G., Zhao, Y. & Sun, L. Bioadhesive microcarriers encapsulated with IL-27 high expressive MSC extracellular vesicles for inflammatory bowel disease treatment. Adv. Sci. 10, e2303349 (2023).
Wirtz, S., Billmeier, U., McHedlidze, T., Blumberg, R. S. & Neurath, M. F. Interleukin-35 mediates mucosal immune responses that protect against T-cell-dependent colitis. Gastroenterology 141, 1875–1886 (2011).
Wang, Y. et al. IL-35 recombinant protein reverses inflammatory bowel disease and psoriasis through regulation of inflammatory cytokines and immune cells. J. Cell. Mol. Med. 22, 1014–1025 (2018).
Wang, J., Tian, M., Li, W. & Hao, F. Preventative delivery of IL-35 by Lactococcus lactis ameliorates DSS-induced colitis in mice. Appl. Microbiol. Biotechnol. 103, 7931–7941 (2019).
Awasthi, A. & Kuchroo, V. K. IL-17A directly inhibits TH1 cells and thereby suppresses development of intestinal inflammation. Nat. Immunol. 10, 568–570 (2009).
Tachibana, M. et al. Ablation of IL-17A leads to severe colitis in IL-10-deficient mice: implications of myeloid-derived suppressor cells and NO production. Int. Immunol. 32, 187–201 (2020).
Lee, J. S. et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity 43, 727–738 (2015).
Ito, R. et al. Involvement of IL-17A in the pathogenesis of DSS-induced colitis in mice. Biochem. Biophys. Res. Commun. 377, 12–16 (2008).
Tang, C. et al. Suppression of IL-17F, but not of IL-17A, provides protection against colitis by inducing Treg cells through modification of the intestinal microbiota. Nat. Immunol. 19, 755–765 (2018).
Leppkes, M. et al. RORγ-expressing TH17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology 136, 257–267 (2009).
Wedebye Schmidt, E. G. et al. TH17 cell induction and effects of IL-17A and IL-17F blockade in experimental colitis. Inflamm. Bowel Dis. 19, 1567–1576 (2013).
Brockmann, L. et al. Molecular and functional heterogeneity of IL-10-producing CD4+ T cells. Nat. Commun. 9, 5457 (2018).
Nguyen, H. D., Aljamaei, H. M. & Stadnyk, A. W. The production and function of endogenous interleukin-10 in intestinal epithelial cells and gut homeostasis. Cell Mol. Gastroenterol. Hepatol. 12, 1343–1352 (2021).
Koelink, P. J. et al. Anti-TNF therapy in IBD exerts its therapeutic effect through macrophage IL-10 signalling. Gut 69, 1053–1063 (2020).
Zigmond, E. et al. Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. Immunity 40, 720–733 (2014).
Uhlig, H. H. et al. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J. Immunol. 177, 5852–5860 (2006).
Neumann, C. et al. c-Maf-dependent Treg cell control of intestinal TH17 cells and IgA establishes host-microbiota homeostasis. Nat. Immunol. 20, 471–481 (2019).
Steidler, L. et al. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science 289, 1352–1355 (2000).
Braat, H., Peppelenbosch, M. P. & Hommes, D. W. Interleukin-10-based therapy for inflammatory bowel disease. Expert. Opin. Biol. Ther. 3, 725–731 (2003).
Zegarra Ruiz, D. F. et al. Microbiota manipulation to increase macrophage IL-10 improves colitis and limits colitis-associated colorectal cancer. Gut Microbes 14, 2119054 (2022).
Fay, N. C. et al. A novel fusion of IL-10 engineered to traffic across intestinal epithelium to treat colitis. J. Immunol. 205, 3191–3204 (2020).
Yang, W. et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat. Commun. 11, 4457 (2020).
Fonseca-Camarillo, G., Furuzawa-Carballeda, J., Llorente, L. & Yamamoto-Furusho, J. K. IL-10- and IL-20-expressing epithelial and inflammatory cells are increased in patients with ulcerative colitis. J. Clin. Immunol. 33, 640–648 (2013).
Chiriac, M. T. et al. IL-20 controls resolution of experimental colitis by regulating epithelial IFN/STAT2 signalling. Gut 73, 282–297 (2023).
Ninnemann, J. et al. TNF hampers intestinal tissue repair in colitis by restricting IL-22 bioavailability. Mucosal Immunol. 15, 698–716 (2022).
Zhu, Q. et al. Epithelial dysfunction is prevented by IL-22 treatment in a Citrobacter rodentium-induced colitis model that shares similarities with inflammatory bowel disease. Mucosal Immunol. 15, 1338–1349 (2022).
Pavlidis, P. et al. Interleukin-22 regulates neutrophil recruitment in ulcerative colitis and is associated with resistance to ustekinumab therapy. Nat. Commun. 13, 5820 (2022).
Wagner, F. et al. Dose escalation randomised study of efmarodocokin alfa in healthy volunteers and patients with ulcerative colitis. Gut 72, 1451–1461 (2023).
Naganuma, M. et al. Efficacy of indigo naturalis in a multicenter randomized controlled trial of patients with ulcerative colitis. Gastroenterology 154, 935–947 (2018).
Heller, F., Fuss, I. J., Nieuwenhuis, E. E., Blumberg, R. S. & Strober, W. Oxazolone colitis, a TH2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity 17, 629–638 (2002).
Fichtner-Feigl, S. et al. IL-13 signaling via IL-13Rɑ2 induces major downstream fibrogenic factors mediating fibrosis in chronic TNBS colitis. Gastroenterology 135, 2003–2013, 2013.e1–e7 (2008).
Fuss, I. J. et al. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical TH2 response in ulcerative colitis. J. Clin. Invest. 113, 1490–1497 (2004).
Rosen, M. J. et al. STAT6 activation in ulcerative colitis: a new target for prevention of IL-13-induced colon epithelial cell dysfunction. Inflamm. Bowel Dis. 17, 2224–2234 (2011).
Reinisch, W. et al. Anrukinzumab, an anti-interleukin 13 monoclonal antibody, in active UC: efficacy and safety from a phase IIa randomised multicentre study. Gut 64, 894–900 (2015).
Danese, S. et al. Tralokinumab for moderate-to-severe UC: a randomised, double-blind, placebo-controlled, phase IIa study. Gut 64, 243–249 (2015).
Perez, L. G. et al. TGF-β signaling in TH17 cells promotes IL-22 production and colitis-associated colon cancer. Nat. Commun. 11, 2608 (2020).
Smyth, D. J. et al. Oral delivery of a functional algal-expressed TGF-β mimic halts colitis in a murine DSS model. J. Biotechnol. 340, 1–12 (2021).
Obata, Y. et al. The epigenetic regulator Uhrf1 facilitates the proliferation and maturation of colonic regulatory T cells. Nat. Immunol. 15, 571–579 (2014).
Fantini, M. C. et al. Transforming growth factor β induced FoxP3+ regulatory T cells suppress TH1 mediated experimental colitis. Gut 55, 671–680 (2006).
Monteleone, G. et al. Mongersen, an oral SMAD7 antisense oligonucleotide, and Crohn’s disease. N. Engl. J. Med. 372, 1104–1113 (2015).
Atreya, R. et al. Cobitolimod for moderate-to-severe, left-sided ulcerative colitis (CONDUCT): a phase 2b randomised, double-blind, placebo-controlled, dose-ranging induction trial. Lancet Gastroenterol. Hepatol. 5, 1063–1075 (2020).
Voskens, C. et al. Autologous regulatory T cell transfer in refractory ulcerative colitis with concomitant primary sclerosing cholangitis. Gut 72, 49–53 (2022).
Desreumaux, P. et al. Safety and efficacy of antigen-specific regulatory T-cell therapy for patients with refractory Crohn’s disease. Gastroenterology 143, 1207–1217.e2 (2012).
Katlinskaya, Y. V. et al. Type I interferons control proliferation and function of the intestinal epithelium. Mol. Cell Biol. 36, 1124–1135 (2016).
Kotredes, K. P., Thomas, B. & Gamero, A. M. The protective role of type I interferons in the gastrointestinal tract. Front. Immunol. 8, 410 (2017).
Katakura, K. et al. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J. Clin. Invest. 115, 695–702 (2005).
Wang, Y. et al. Type I interferons for induction of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2015, CD006790 (2015).
Kamada, N. et al. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-γ axis. J. Clin. Invest. 118, 2269–2280 (2008).
Wang, F. et al. IFN-γ-induced TNFR2 expression is required for TNF-dependent intestinal epithelial barrier dysfunction. Gastroenterology 131, 1153–1163 (2006).
Walrath, T. et al. IFN-γ and IL-17A regulate intestinal crypt production of CXCL10 in the healthy and inflamed colon. Am. J. Physiol. Gastrointest. Liver Physiol. 318, G479–G489 (2020).
Langer, V. et al. IFN-γ drives inflammatory bowel disease pathogenesis through VE-cadherin-directed vascular barrier disruption. J. Clin. Invest. 129, 4691–4707 (2019).
Reinisch, W. et al. Fontolizumab in moderate to severe Crohn’s disease: a phase 2, randomized, double-blind, placebo-controlled, multiple-dose study. Inflamm. Bowel Dis. 16, 233–242 (2010).
Chiriac, M. T. et al. Activation of epithelial signal transducer and activator of transcription 1 by interleukin 28 controls mucosal healing in mice with colitis and is increased in mucosa of patients with inflammatory bowel disease. Gastroenterology 153, 123–138.e8 (2017).
Gunther, C. et al. Interferon λ promotes paneth cell death via STAT1 signaling in mice and is increased in inflamed ileal tissues of patients with Crohn’s disease. Gastroenterology 157, 1310–1322 e1313 (2019).
Xu, P. et al. Interleukin-28A induces epithelial barrier dysfunction in CD patient-derived intestinal organoids. Am. J. Physiol. Gastrointest. Liver Physiol. 320, G689–G699 (2021).
Tuzlak, S. et al. Repositioning TH cell polarization from single cytokines to complex help. Nat. Immunol. 22, 1210–1217 (2021).
Jacobse, J., Li, J., Rings, E., Samsom, J. N. & Goettel, J. A. Intestinal regulatory T cells as specialized tissue-restricted immune cells in intestinal immune homeostasis and disease. Front. Immunol. 12, 716499 (2021).
Ueno, A. et al. TH17 plasticity and its relevance to inflammatory bowel disease. J. Autoimmun. 87, 38–49 (2018).
Ueno, A., Ghosh, A., Hung, D., Li, J. & Jijon, H. TH17 plasticity and its changes associated with inflammatory bowel disease. World J. Gastroenterol. 21, 12283–12295 (2015).
Li, J. et al. Crossover subsets of CD4+ T lymphocytes in the intestinal lamina propria of patients with Crohn’s disease and ulcerative colitis. Dig. Dis. Sci. 62, 2357–2368 (2017).
Kiner, E. et al. Gut CD4+ T cell phenotypes are a continuum molded by microbes, not by TH archetypes. Nat. Immunol. 22, 216–228 (2021).
Schmitt, H. et al. Expansion of IL-23 receptor bearing TNFR2+ T cells is associated with molecular resistance to anti-TNF therapy in Crohn’s disease. Gut 68(5):814-828. (2019).
Danese, S. & Fiocchi, C. Ulcerative colitis. N. Engl. J. Med. 365, 1713–1725 (2011).
Solitano, V. et al. Advanced combination treatment with biologic agents and novel small molecule drugs for inflammatory bowel disease. Gastroenterol. Hepatol. 19, 251–263 (2023).
Colombel, J. F. et al. Vedolizumab, adalimumab, and methotrexate combination therapy in Crohn’s disease (EXPLORER). Clin. Gastroenterol. Hepatol. :S1542-3565(23)00746-2. https://doi.org/10.1016/j.cgh.2023.09.010 (2023).
Feagan, B. G. et al. Guselkumab plus golimumab combination therapy versus guselkumab or golimumab monotherapy in patients with ulcerative colitis (VEGA): a randomised, double-blind, controlled, phase 2, proof-of-concept trial. Lancet Gastroenterol. Hepatol. 8, 307–320 (2023).
Sandborn, W. J., Su, C. & Panes, J. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 377, 496–497 (2017).
Feagan, B. G. et al. Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): a phase 2b/3 double-blind, randomised, placebo-controlled trial. Lancet 397, 2372–2384 (2021).
Danese, S. et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet 399, 2113–2128 (2022).
Sands, B. E. et al. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N. Engl. J. Med. 381, 1215–1226 (2019).
Sands, B. E. et al. Ustekinumab versus adalimumab for induction and maintenance therapy in biologic-naive patients with moderately to severely active Crohn’s disease: a multicentre, randomised, double-blind, parallel-group, phase 3b trial. Lancet 399, 2200–2211 (2022).
Peyrin-Biroulet, L. SEQUENCE: Risankizumab doubles endoscopic remission rates compared with ustekinumab in CD. MediCom https://conferences.medicom-publishers.com/specialisation/gastroenterology/uegw-2023/sequence-risankizumab-doubles-endoscopic-remission-rates-compared-with-ustekinumab-in-cd/ (2023).
Czarnewski, P. et al. Conserved transcriptomic profile between mouse and human colitis allows unsupervised patient stratification. Nat. Commun. 10, 2892 (2019).
Chiabai, M. J. et al. Mucosal delivery of Lactococcus lactis carrying an anti-TNF scFv expression vector ameliorates experimental colitis in mice. BMC Biotechnol. 19, 38 (2019).
Nurbhai, S. et al. Oral anti-tumour necrosis factor domain antibody v565 provides high intestinal concentrations, and reduces markers of inflammation in ulcerative colitis patients. Sci. Rep. 9, 14042 (2019).
Roberts, K. J. et al. Preclinical development of a bispecific TNFɑ/IL-23 neutralising domain antibody as a novel oral treatment for inflammatory bowel disease. Sci. Rep. 11, 19422 (2021).
Louis, T. J., Qasem, A. & Naser, S. A. Attenuation of excess TNF-ɑ release in Crohn’s disease by silencing of iRHOMs 1/2 and the restoration of TGF-β mediated immunosuppression through modulation of TACE trafficking. Front. Immunol. 13, 887830 (2022).
Deckers, J. et al. Engineering cytokine therapeutics. Nat. Rev. Bioeng. 1, 286–303 (2023).
Saxton, R. A. et al. The tissue protective functions of interleukin-22 can be decoupled from pro-inflammatory actions through structure-based design. Immunity 54, 660–672.e9 (2021).
Saxton, R. A. et al. Structure-based decoupling of the pro- and anti-inflammatory functions of interleukin-10. Science 371, eabc8433 (2021).
Khoryati, L. et al. An IL-2 mutein engineered to promote expansion of regulatory T cells arrests ongoing autoimmunity in mice. Sci. Immunol. 5, eaba5264 (2020).
Franks, S. E. et al. Exploiting docetaxel-induced tumor cell necrosis with tumor targeted delivery of IL-12. Cancer Immunol. Immunother. 72, 2783–2797 (2023).
Zhang, L. & Pohl, C. S. Imaging the alternatively spliced D domain of tenascin C in preclinical models of inflammatory bowel disease. Research Square https://assets.researchsquare.com/files/rs-984872/v1/7f2b6767-36bf-4f01-944f-94cb36045985.pdf?c=1638428869 (2023).
Apolit, C. et al. ABX464 (obefazimod) upregulates miR-124 to reduce proinflammatory markers in inflammatory bowel diseases. Clin. Transl. Gastroenterol. 14, e00560 (2023).
Vermeire, S. et al. ABX464 (obefazimod) for moderate-to-severe, active ulcerative colitis: a phase 2b, double-blind, randomised, placebo-controlled induction trial and 48 week, open-label extension. Lancet Gastroenterol. Hepatol. 7, 1024–1035 (2022).
Doherty, M. K. et al. Fecal microbiota signatures are associated with response to ustekinumab therapy among Crohn’s disease patients. mBio 9, e02120–e02127 (2018).
Rajca, S. et al. Alterations in the intestinal microbiome (dysbiosis) as a predictor of relapse after infliximab withdrawal in Crohn’s disease. Inflamm. Bowel Dis. 20, 978–986 (2014).
Zheng, H. B. Application of single-cell omics in inflammatory bowel disease. World J. Gastroenterol. 29, 4397–4404 (2023).
Schett, G., McInnes, I. B. & Neurath, M. F. Reframing immune-mediated inflammatory diseases through signature cytokine hubs. N. Engl. J. Med. 385, 628–639 (2021).
Acknowledgements
The author thanks C. Becker, S. Zundler, M. Waldner, T. Rath and R Atreya for helpful discussions. The research of M.F.N. has been supported by the IZKF Erlangen and the Deutsche Forschungsgemeinschaft (DFG) SFB1181, FOR2438, FOR5024, DFG Sachbeihilfe (IL-20 and STAT2 in IBD) and TRR241.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
M.F.N. has served as an adviser for Abbvie, Janssen, Boehringer, MSD, UCB, PPM, Pentax and Takeda.
Peer review
Peer review information
Nature Reviews Immunology thanks H. Tilg and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Neurath, M.F. Strategies for targeting cytokines in inflammatory bowel disease. Nat Rev Immunol (2024). https://doi.org/10.1038/s41577-024-01008-6
Accepted:
Published:
DOI: https://doi.org/10.1038/s41577-024-01008-6