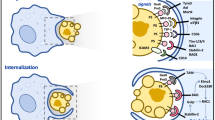
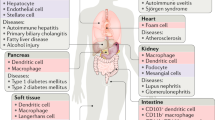
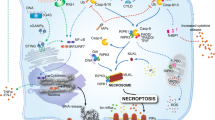
Abstract
Cell death occurs when a pathogen invades a host organism or the organism is subjected to sterile injury. Thus, cell death is often closely associated with the induction of an immune response. Furthermore, cell death can occur as a consequence of the immune response and precedes the tissue renewal and repair responses that are initiated by innate immune cells during resolution of an immune response. Beyond immunity, cell death is required for development, morphogenesis and homeostasis. How can such a ubiquitous event as cell death trigger such a wide range of context-specific effector responses? Dying cells are sensed by innate immune cells using specialized receptors and phagocytosed through a process termed efferocytosis. Here, we outline a general principle whereby signals within the dead cell as well as the environment are integrated by specific efferocytes to define the appropriate effector response.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lowin, B., Peitsch, M. C. & Tschopp, J. Perforin and granzymes: crucial effector molecules in cytolytic T lymphocyte and natural killer cell-mediated cytotoxicity. Curr. Top. Microbiol. Immunol. 198, 1–24 (1995).
Gordon, S. The macrophage: past, present and future. Eur. J. Immunol. 37, S9–S17 (2007).
Ahmed, R. & Gray, D. Immunological memory and protective immunity: understanding their relation. Science 272, 54–60 (1996).
Swain, S. L. et al. From naive to memory T cells. Immunol. Rev. 150, 143–167 (1996).
Martinez, J. Prix fixe: efferocytosis as a four-course meal. Curr. Top. Microbiol. Immunol. 403, 1–36 (2017).
Bratosin, D. et al. Cellular and molecular mechanisms of senescent erythrocyte phagocytosis by macrophages. A review. Biochimie 80, 173–195 (1998).
Voss, A. K. & Strasser, A. The essentials of developmental apoptosis. F1000Res https://doi.org/10.12688/f1000research.21571.1 (2020).
Hopkinson-Woolley, J., Hughes, D., Gordon, S. & Martin, P. Macrophage recruitment during limb development and wound healing in the embryonic and foetal mouse. J. Cell Sci. 107, 1159–1167 (1994).
Theofilopoulos, A. N. & Dixon, F. J. Murine models of systemic lupus erythematosus. Adv. Immunol. 37, 269–390 (1985).
Andrews, B. S. et al. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J. Exp. Med. 148, 1198–1215 (1978).
Roths, J. B., Murphy, E. D. & Eicher, E. M. A new mutation, gld, that produces lymphoproliferation and autoimmunity in C3H/HeJ mice. J. Exp. Med. 159, 1–20 (1984).
Fisher, G. H. et al. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell 81, 935–946 (1995).
Chun, H. J. et al. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature 419, 395–399 (2002).
Wang, J. et al. Inherited human caspase 10 mutations underlie defective lymphocyte and dendritic cell apoptosis in autoimmune lymphoproliferative syndrome type II. Cell 98, 47–58 (1999).
Rieux-Laucat, F. et al. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science 268, 1347–1349 (1995).
Bouillet, P. et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 286, 1735–1738 (1999).
Cox, G., Crossley, J. & Xing, Z. Macrophage engulfment of apoptotic neutrophils contributes to the resolution of acute pulmonary inflammation in vivo. Am. J. Respir. Cell Mol. Biol. 12, 232–237 (1995).
Bosurgi, L. et al. Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science 356, 1072–1076 (2017). This study is the first to show that the integration of IL-4/IL-13 cytokine receptor signalling with sensing of apoptotic neutrophils is required for the induction of tissue repair responses in macrophages.
Torchinsky, M. B., Garaude, J., Martin, A. P. & Blander, J. M. Innate immune recognition of infected apoptotic cells directs TH17 cell differentiation. Nature 458, 78–82 (2009). This paper shows that the recognition of infected apoptotic cells leads to the induction of TH17 cell responses, whereas the response to apoptotic cells without microbial signals induces the differentiation of Treg cells.
Brereton, C. F. & Blander, J. M. The unexpected link between infection-induced apoptosis and a TH17 immune response. J. Leukoc. Biol. 89, 565–576 (2011).
Mebius, R. E. & Kraal, G. Structure and function of the spleen. Nat. Rev. Immunol. 5, 606–616 (2005).
de Back, D. Z., Kostova, E. B., van Kraaij, M., van den Berg, T. K. & van Bruggen, R. Of macrophages and red blood cells; a complex love story. Front. Physiol. 5, 9 (2014).
Yamaguchi, Y. & Miura, M. Programmed cell death in neurodevelopment. Dev. Cell 32, 478–490 (2015).
Kuan, C. Y. et al. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron 22, 667–676 (1999).
De Zio, D., Giunta, L., Corvaro, M., Ferraro, E. & Cecconi, F. Expanding roles of programmed cell death in mammalian neurodevelopment. Semin. Cell Dev. Biol. 16, 281–294 (2005).
Yoshida, H. et al. Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell 94, 739–750 (1998).
Cecconi, F., Alvarez-Bolado, G., Meyer, B. I., Roth, K. A. & Gruss, P. Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell 94, 727–737 (1998).
Brinkmann, V. et al. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 (2004). This is the first study describing the formation of neutrophil extracellular traps, an effector function of NETosis.
Fink, S. L. & Cookson, B. T. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell. Microbiol. 8, 1812–1825 (2006).
Fadeel, B. Programmed cell clearance. Cell. Mol. Life Sci. 60, 2575–2585 (2003).
Franc, N. C. Phagocytosis of apoptotic cells in mammals, Caenorhabditis elegans and Drosophila melanogaster: molecular mechanisms and physiological consequences. Front. Biosci. 7, d1298–d1313 (2002).
Arandjelovic, S. & Ravichandran, K. S. Phagocytosis of apoptotic cells in homeostasis. Nat. Immunol. 16, 907–917 (2015).
Blander, J. M. The many ways tissue phagocytes respond to dying cells. Immunol. Rev. 277, 158–173 (2017).
Rogers, C. et al. Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat. Commun. 10, 1689 (2019).
Davidovich, P., Kearney, C. J. & Martin, S. J. Inflammatory outcomes of apoptosis, necrosis and necroptosis. Biol. Chem. 395, 1163–1171 (2014).
Lammert, C. R. et al. AIM2 inflammasome surveillance of DNA damage shapes neurodevelopment. Nature 580, 647–652 (2020). This study reports the requirement for AIM2 inflammasome and gasdermin D, but not IL-1 or IL-18, in the response to genotoxic stress in neurons during development.
Kleinclauss, F. et al. Intravenous apoptotic spleen cell infusion induces a TGF-β-dependent regulatory T-cell expansion. Cell Death Differ. 13, 41–52 (2006).
Nagata, S. Apoptosis and clearance of apoptotic cells. Annu. Rev. Immunol. 36, 489–517 (2018).
Martin, S. J. et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J. Exp. Med. 182, 1545–1556 (1995). This landmark study reports the early externalization of PtdSer in a wide array of mouse and human cells undergoing apoptosis.
Maeda, Y., Shiratsuchi, A., Namiki, M. & Nakanishi, Y. Inhibition of sperm production in mice by annexin V microinjected into seminiferous tubules: possible etiology of phagocytic clearance of apoptotic spermatogenic cells and male infertility. Cell Death Differ. 9, 742–749 (2002).
Franc, N. C., Dimarcq, J. L., Lagueux, M., Hoffmann, J. & Ezekowitz, R. A. Croquemort, a novel Drosophila hemocyte/macrophage receptor that recognizes apoptotic cells. Immunity 4, 431–443 (1996).
Lang, R. A. & Bishop, J. M. Macrophages are required for cell death and tissue remodeling in the developing mouse eye. Cell 74, 453–462 (1993).
Diez-Roux, G. & Lang, R. A. Macrophages induce apoptosis in normal cells in vivo. Development 124, 3633–3638 (1997).
Hoeppner, D. J., Hengartner, M. O. & Schnabel, R. Engulfment genes cooperate with ced-3 to promote cell death in Caenorhabditis elegans. Nature 412, 202–206 (2001).
Reddien, P. W., Cameron, S. & Horvitz, H. R. Phagocytosis promotes programmed cell death in C. elegans. Nature 412, 198–202 (2001). Together with Hoeppner et al. (2001), this paper presents the discovery that cells normally destined to die survive in C. elegans carrying mutations in engulfment genes.
Galluzzi, L. et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 25, 486–541 (2018).
Bergsbaken, T., Fink, S. L. & Cookson, B. T. Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 7, 99–109 (2009).
Sulston, J. E., Schierenberg, E., White, J. G. & Thomson, J. N. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100, 64–119 (1983).
Juncadella, I. J. et al. Apoptotic cell clearance by bronchial epithelial cells critically influences airway inflammation. Nature 493, 547–551 (2013). This manuscript identifies bronchial epithelial cells as phagocytes for neighbouring apoptotic epithelial cells and inducers of anti-inflammatory responses.
Han, C. Z. et al. Macrophages redirect phagocytosis by non-professional phagocytes and influence inflammation. Nature 539, 570–574 (2016). This study reports the ability of engulfing macrophages to regulate the phagocytic capacity and inflammatory response of epithelial cells.
Hall, S. E., Savill, J. S., Henson, P. M. & Haslett, C. Apoptotic neutrophils are phagocytosed by fibroblasts with participation of the fibroblast vitronectin receptor and involvement of a mannose/fucose-specific lectin. J. Immunol. 153, 3218–3227 (1994).
Ramirez-Ortiz, Z. G. et al. The scavenger receptor SCARF1 mediates the clearance of apoptotic cells and prevents autoimmunity. Nat. Immunol. 14, 917–926 (2013).
Kumar, S. & Birge, R. B. Efferocytosis. Curr. Biol. 26, R558–R559 (2016).
Lu, Z. et al. Phagocytic activity of neuronal progenitors regulates adult neurogenesis. Nat. Cell Biol. 13, 1076–1083 (2011).
Wu, H. H. et al. Glial precursors clear sensory neuron corpses during development via Jedi-1, an engulfment receptor. Nat. Neurosci. 12, 1534–1541 (2009).
Nguyen, J. V. et al. Myelination transition zone astrocytes are constitutively phagocytic and have synuclein dependent reactivity in glaucoma. Proc. Natl Acad. Sci. USA 108, 1176–1181 (2011).
Morizawa, Y. M. et al. Reactive astrocytes function as phagocytes after brain ischemia via ABCA1-mediated pathway. Nat. Commun. 8, 28 (2017).
Loov, C., Hillered, L., Ebendal, T. & Erlandsson, A. Engulfing astrocytes protect neurons from contact-induced apoptosis following injury. PLoS ONE 7, e33090 (2012).
Iram, T. et al. Megf10 is a receptor for C1Q that mediates clearance of apoptotic cells by astrocytes. J. Neurosci. 36, 5185–5192 (2016).
Damisah, E. C. et al. Astrocytes and microglia play orchestrated roles and respect phagocytic territories during neuronal corpse removal in vivo. Sci. Adv. 6, eaba3239 (2020). This study makes use of photochemical induction of death in a single neuron in the mouse brain to report the ability of astrocytes to engulf small dendritic apoptotic bodies, whereas microglia engulf the soma and apical dendrites.
Reemst, K., Noctor, S. C., Lucassen, P. J. & Hol, E. M. The indispensable roles of microglia and astrocytes during brain development. Front. Hum. Neurosci. 10, 566 (2016).
Blaschke, A. J., Staley, K. & Chun, J. Widespread programmed cell death in proliferative and postmitotic regions of the fetal cerebral cortex. Development 122, 1165–1174 (1996).
Wong, F. K. & Marin, O. Developmental cell death in the cerebral cortex. Annu. Rev. Cell Dev. Biol. 35, 523–542 (2019).
Morioka, S., Maueroder, C. & Ravichandran, K. S. Living on the edge: efferocytosis at the interface of homeostasis and pathology. Immunity 50, 1149–1162 (2019).
Cummings, R. J. et al. Different tissue phagocytes sample apoptotic cells to direct distinct homeostasis programs. Nature 539, 565–569 (2016). This study defines the transcriptional changes of distinct subsets of intestinal dendritic cells and macrophages as a consequence of the homeostatic removal of apoptotic epithelial cells.
Savill, J. S. et al. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J. Clin. Invest. 83, 865–875 (1989).
Boulter, L. et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat. Med. 18, 572–579 (2012).
Duffield, J. S. et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J. Clin. Invest. 115, 56–65 (2005).
Lucas, T. et al. Differential roles of macrophages in diverse phases of skin repair. J. Immunol. 184, 3964–3977 (2010).
Gumienny, T. L. & Hengartner, M. O. How the worm removes corpses: the nematode C. elegans as a model system to study engulfment. Cell Death Differ. 8, 564–568 (2001).
A-Gonzalez, N. et al. Phagocytosis imprints heterogeneity in tissue-resident macrophages. J. Exp. Med. 214, 1281–1296 (2017). This study identifies distinct types of receptors, opsonins and transcription factors in the phagocytosis of apoptotic cells by tissue-resident macrophages, underscoring the heterogeneity of this process across multiple tissues.
Mass, E. et al. Specification of tissue-resident macrophages during organogenesis. Science 353, aaf4238 (2016).
Tasdemir-Yilmaz, O. E. & Freeman, M. R. Astrocytes engage unique molecular programs to engulf pruned neuronal debris from distinct subsets of neurons. Genes Dev. 28, 20–33 (2014).
D’Cruz, P. M. et al. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum. Mol. Genet. 9, 645–651 (2000).
Chen, Y. et al. Functions of TAM RTKs in regulating spermatogenesis and male fertility in mice. Reproduction 138, 655–666 (2009).
Duncan, J. L. et al. An RCS-like retinal dystrophy phenotype in mer knockout mice. Invest. Ophthalmol. Vis. Sci. 44, 826–838 (2003).
Penberthy, K. K. et al. Context-dependent compensation among phosphatidylserine-recognition receptors. Sci. Rep. 7, 14623 (2017).
Green, D. R., Ferguson, T., Zitvogel, L. & Kroemer, G. Immunogenic and tolerogenic cell death. Nat. Rev. Immunol. 9, 353–363 (2009).
Ferguson, T. A., Choi, J. & Green, D. R. Armed response: how dying cells influence T-cell functions. Immunol. Rev. 241, 77–88 (2011).
Jorgensen, I., Zhang, Y., Krantz, B. A. & Miao, E. A. Pyroptosis triggers pore-induced intracellular traps (PITs) that capture bacteria and lead to their clearance by efferocytosis. J. Exp. Med. 213, 2113–2128 (2016).
Jorgensen, I. & Miao, E. A. Pyroptotic cell death defends against intracellular pathogens. Immunol. Rev. 265, 130–142 (2015).
Xu, Y. et al. A bacterial effector reveals the V-ATPase–ATG16L1 axis that initiates xenophagy. Cell 178, 552–566 (2019).
Dinarello, C. A. IL-18: a TH1-inducing, proinflammatory cytokine and new member of the IL-1 family. J. Allergy. Clin. Immunol. 103, 11–24 (1999).
Veldhoen, M., Hocking, R. J., Atkins, C. J., Locksley, R. M. & Stockinger, B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24, 179–189 (2006).
Sutton, C. E. et al. Interleukin-1 and IL-23 induce innate IL-17 production from γδ T cells, amplifying TH17 responses and autoimmunity. Immunity 31, 331–341 (2009).
Rathinam, V. A. & Fitzgerald, K. A. Inflammasome complexes: emerging mechanisms and effector functions. Cell 165, 792–800 (2016).
Lee, S., Hirohama, M., Noguchi, M., Nagata, K. & Kawaguchi, A. Influenza A virus infection triggers pyroptosis and apoptosis of respiratory epithelial cells through the type I interferon signaling pathway in a mutually exclusive manner. J. Virol. 92, e00396–00418 (2018).
Tan, M. S. et al. Amyloid-β induces NLRP1-dependent neuronal pyroptosis in models of Alzheimer’s disease. Cell Death Dis. 5, e1382 (2014).
Szabo, G. & Petrasek, J. Inflammasome activation and function in liver disease. Nat. Rev. Gastroenterol. Hepatol. 12, 387–400 (2015).
Liu, G. et al. High mobility group protein-1 inhibits phagocytosis of apoptotic neutrophils through binding to phosphatidylserine. J. Immunol. 181, 4240–4246 (2008).
Friggeri, A. et al. HMGB1 inhibits macrophage activity in efferocytosis through binding to the αvβ3-integrin. Am. J. Physiol. Cell. Physiol. 299, C1267–C1276 (2010).
Tiefenthaler, M. et al. Increased lactate production follows loss of mitochondrial membrane potential during apoptosis of human leukaemia cells. Br. J. Haematol. 114, 574–580 (2001).
Colegio, O. R. et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 513, 559–563 (2014).
Greenlee-Wacker, M. C. et al. Phagocytosis of Staphylococcus aureus by human neutrophils prevents macrophage efferocytosis and induces programmed necrosis. J. Immunol. 192, 4709–4717 (2014).
Newton, K. et al. Activity of caspase-8 determines plasticity between cell death pathways. Nature 575, 679–682 (2019).
Fritsch, M. et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature 575, 683–687 (2019). Together with Newton et al. (Nature 575, 2019), this study reveals the plasticity between and hierarchy across different cell death modalities when specific executionary mediators of cell death are inhibited.
Shan, B., Pan, H., Najafov, A. & Yuan, J. Necroptosis in development and diseases. Genes Dev. 32, 327–340 (2018).
Newton, K., Sun, X. & Dixit, V. M. Kinase RIP3 is dispensable for normal NF-κBs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol. Cell. Biol. 24, 1464–1469 (2004).
Wu, J. et al. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res. 23, 994–1006 (2013).
Polykratis, A. et al. Cutting edge: RIPK1 kinase inactive mice are viable and protected from TNF-induced necroptosis in vivo. J. Immunol. 193, 1539–1543 (2014).
Kaiser, W. J. et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471, 368–372 (2011).
Oberst, A. et al. Catalytic activity of the caspase-8-FLIPL complex inhibits RIPK3-dependent necrosis. Nature 471, 363–367 (2011).
Alvarez-Diaz, S. et al. The pseudokinase MLKL and the kinase RIPK3 have distinct roles in autoimmune disease caused by loss of death-receptor-induced apoptosis. Immunity 45, 513–526 (2016).
Chen, C. C. et al. Organ-level quorum sensing directs regeneration in hair stem cell populations. Cell 161, 277–290 (2015).
Wang, Y. et al. Mitochondrial fission promotes the continued clearance of apoptotic cells by macrophages. Cell 171, 331–345 (2017). This study identifies a metabolic pathway required for sequential phagocytosis.
Sisson, T. H. et al. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 181, 254–263 (2010).
Kim, K. K. et al. Efferocytosis of apoptotic alveolar epithelial cells is sufficient to initiate lung fibrosis. Cell Death Dis. 9, 1056 (2018).
Martin, S. J. Cell death and inflammation: the case for IL-1 family cytokines as the canonical DAMPs of the immune system. FEBS J. 283, 2599–2615 (2016).
Arpaia, N. et al. A distinct function of regulatory T cells in tissue protection. Cell 162, 1078–1089 (2015).
Greter, M. et al. Stroma-derived interleukin-34 controls the development and maintenance of Langerhans cells and the maintenance of microglia. Immunity 37, 1050–1060 (2012).
Merad, M., Ginhoux, F. & Collin, M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat. Rev. Immunol. 8, 935–947 (2008).
Igyarto, B. Z. et al. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity 35, 260–272 (2011).
Gyorki, D. E., Asselin-Labat, M. L., van Rooijen, N., Lindeman, G. J. & Visvader, J. E. Resident macrophages influence stem cell activity in the mammary gland. Breast Cancer Res. 11, R62 (2009).
Gouon-Evans, V., Rothenberg, M. E. & Pollard, J. W. Postnatal mammary gland development requires macrophages and eosinophils. Development 127, 2269–2282 (2000).
Monks, J., Smith-Steinhart, C., Kruk, E. R., Fadok, V. A. & Henson, P. M. Epithelial cells remove apoptotic epithelial cells during post-lactation involution of the mouse mammary gland. Biol. Reprod. 78, 586–594 (2008).
O’Brien, J., Martinson, H., Durand-Rougely, C. & Schedin, P. Macrophages are crucial for epithelial cell death and adipocyte repopulation during mammary gland involution. Development 139, 269–275 (2012).
Minutti, C. M. et al. Local amplifiers of IL-4Rα-mediated macrophage activation promote repair in lung and liver. Science 356, 1076–1080 (2017).
Sommerfeld, S. D. et al. Interleukin-36γ-producing macrophages drive IL-17-mediated fibrosis. Sci. Immunol. 4, aax4783 (2019).
Gosselin, D. et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell 159, 1327–1340 (2014).
Sakai, M. et al. Liver-derived signals sequentially reprogram myeloid enhancers to initiate and maintain Kupffer cell identity. Immunity 51, 655–670 (2019).
Cohen, M. et al. Lung single-cell signaling interaction map reveals basophil role in macrophage imprinting. Cell 175, 1031–1044 (2018).
Buechler, M. B. et al. A stromal niche defined by expression of the transcription factor WT1 mediates programming and homeostasis of cavity-resident macrophages. Immunity 51, 119–130 (2019).
Svedberg, F. R. et al. The lung environment controls alveolar macrophage metabolism and responsiveness in type 2 inflammation. Nat. Immunol. 20, 571–580 (2019).
van Beek, A. A., Van den Bossche, J., Mastroberardino, P. G., de Winther, M. P. J. & Leenen, P. J. M. Metabolic alterations in aging macrophages: ingredients for inflammaging? Trends Immunol. 40, 113–127 (2019).
Frisch, B. J. et al. Aged marrow macrophages expand platelet-biased hematopoietic stem cells via interleukin-1B. JCI Insight 5, e124213 (2019).
Keenan, C. R. & Allan, R. S. Epigenomic drivers of immune dysfunction in aging. Aging Cell 18, e12878 (2019).
Gerlach, B. D. et al. Resolvin D1 promotes the targeting and clearance of necroptotic cells. Cell Death Differ. 27, 525–539 (2019).
Newton, K. et al. Cleavage of RIPK1 by caspase-8 is crucial for limiting apoptosis and necroptosis. Nature 574, 428–431 (2019).
Riegler, A. N., Brissac, T., Gonzalez-Juarbe, N. & Orihuela, C. J. Necroptotic cell death promotes adaptive immunity against colonizing pneumococci. Front. Immunol. 10, 615 (2019).
Cho, Y. S. et al. Phosphorylation-driven assembly of the RIP1–RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137, 1112–1123 (2009).
Molnar, T. et al. Current translational potential and underlying molecular mechanisms of necroptosis. Cell Death Dis. 10, 860 (2019).
Li, S., Ning, L. G., Lou, X. H. & Xu, G. Q. Necroptosis in inflammatory bowel disease and other intestinal diseases. World J. Clin. Cases 6, 745–752 (2018).
Hermetet, F. et al. Efferocytosis of apoptotic human papillomavirus-positive cervical cancer cells by human primary fibroblasts. Biol. Cell 108, 189–204 (2016).
Roberts, E. W. et al. Critical role for CD103+/CD141+ dendritic cells bearing CCR7 for tumor antigen trafficking and priming of T cell immunity in melanoma. Cancer Cell 30, 324–336 (2016).
Pober, J. S. et al. Lymphocytes recognize human vascular endothelial and dermal fibroblast Ia antigens induced by recombinant immune interferon. Nature 305, 726–729 (1983).
Boots, A. M., Wimmers-Bertens, A. J. & Rijnders, A. W. Antigen-presenting capacity of rheumatoid synovial fibroblasts. Immunology 82, 268–274 (1994).
Rieder, F., Brenmoehl, J., Leeb, S., Scholmerich, J. & Rogler, G. Wound healing and fibrosis in intestinal disease. Gut 56, 130–139 (2007).
Martinez, F. J. et al. Idiopathic pulmonary fibrosis. Nat. Rev. Dis. Prim. 3, 17074 (2017).
Hernandez-Gea, V. & Friedman, S. L. Pathogenesis of liver fibrosis. Annu. Rev. Pathol. 6, 425–456 (2011).
Rowe, S. M., Miller, S. & Sorscher, E. J. Cystic fibrosis. N. Engl. J. Med. 352, 1992–2001 (2005).
Ratjen, F. & Doring, G. Cystic fibrosis. Lancet 361, 681–689 (2003).
Elraiyah, T. et al. A systematic review and meta-analysis of debridement methods for chronic diabetic foot ulcers. J. Vasc. Surg. 63, 37S–45S (2016).
Cornell, R. S., Meyr, A. J., Steinberg, J. S. & Attinger, C. E. Debridement of the noninfected wound. J. Vasc. Surg. 52, 31S–36S (2010).
Magnus, T., Chan, A., Linker, R. A., Toyka, K. V. & Gold, R. Astrocytes are less efficient in the removal of apoptotic lymphocytes than microglia cells: implications for the role of glial cells in the inflamed central nervous system. J. Neuropathol. Exp. Neurol. 61, 760–766 (2002).
Acknowledgements
C.V.R. and S.G. acknowledge M. K. Basu for analyses and discussions of single-cell RNA-sequencing data. T.D.H. acknowledges M. Chalfant for insightful discussions. This work was supported by grants from the National Institutes of Health (NIH-NIAID R01 AI089824 and NIH-NCI R01 CA212376) and the Kenneth Rainin Foundation. C.V.R is a Howard Hughes Medical Institute Faculty Scholar.
Author information
Authors and Affiliations
Contributions
T.D.H conceived and developed the mathematical representation. C.V.R and S.G. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Immunology thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
VirtualCytometry: https://www.grnpedia.org/cytometry
Glossary
- Necrosome complex
-
An amyloid signalling complex assembled upon interaction and activation (phosphorylation) of receptor-interacting serine/threonine protein kinase 3 (RIPK3) and RIPK1. The necrosome leads to the phosphorylation of mixed lineage kinase domain-like protein (MLKL) and induction of necroptosis.
- LC3-associated phagocytosis
-
(LAP). A form of phagocytosis during which the canonical autophagy protein LC3 is conjugated to the phagosome to form the LAPosome. LC3 conjugation is crucial for phagosome maturation and acidification, through fusion with lysosomes, and the degradation of cargo.
Rights and permissions
About this article
Cite this article
Rothlin, C.V., Hille, T.D. & Ghosh, S. Determining the effector response to cell death. Nat Rev Immunol 21, 292–304 (2021). https://doi.org/10.1038/s41577-020-00456-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-020-00456-0
This article is cited by
-
Cell death and senescence
Journal of Translational Medicine (2023)
-
Targeted immunotherapy for glioblastoma involving whole tumor-derived autologous cells in the upfront setting after craniotomy
Journal of Neuro-Oncology (2023)
-
Single-cell sequencing and bulk RNA data reveal the tumor microenvironment infiltration characteristics of disulfidptosis related genes in breast cancer
Journal of Cancer Research and Clinical Oncology (2023)
-
Construction of a Novel Disulfidptosis-Related lncRNA Prognostic Signature in Pancreatic Cancer
Molecular Biotechnology (2023)
-
Apoptotic cell death in disease—Current understanding of the NCCD 2023
Cell Death & Differentiation (2023)