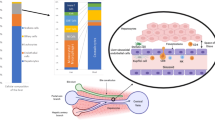
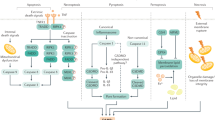
Abstract
In the past 40 years, liver transplantation has evolved from a high-risk procedure to one that offers high success rates for reversal of liver dysfunction and excellent patient and graft survival. The liver is the most tolerogenic of transplanted organs; indeed, immunosuppressive therapy can be completely withdrawn without rejection of the graft in carefully selected, stable long-term liver recipients. However, in other recipients, chronic allograft injury, late graft failure and the adverse effects of anti-rejection therapy remain important obstacles to improved success. The liver has a unique composition of parenchymal and immune cells that regulate innate and adaptive immunity and that can promote antigen-specific tolerance. Although the mechanisms underlying liver transplant tolerance are not well understood, important insights have been gained into how the local microenvironment, hepatic immune cells and specific molecular pathways can promote donor-specific tolerance. These insights provide a basis for the identification of potential clinical biomarkers that might correlate with tolerance or rejection and for the development of novel therapeutic targets. Innovative approaches aimed at promoting immunosuppressive drug minimization or withdrawal include the adoptive transfer of donor-derived or recipient-derived regulatory immune cells to promote liver transplant tolerance. In this Review, we summarize and discuss these developments and their implications for liver transplantation.
Key points
-
Liver transplant tolerance occurs in animals without immunosuppressive therapy. In humans, donor–recipient histocompatibility is not considered important for graft survival; in carefully selected patients, complete immunosuppression withdrawal is currently possible.
-
Parenchymal and non-parenchymal cells, particularly those of the innate and adaptive immune system, seem to have key roles in promoting a tolerogenic environment within the liver via various mechanisms.
-
Donor-derived haematopoietic cells trafficking to the host, exhaustion or death of graft-infiltrating donor-reactive T cells, and regulatory dendritic cell and regulatory T cell responses have been implicated in experimental liver transplant tolerance.
-
Early (<3 months) acute cellular rejection might not harm the graft in the long term; patients might, however, harbour latent T cell-mediated rejection and/or fibrosis. The pathogenicity of donor-specific antibodies remains uncertain.
-
Graft biopsy assessment and intra-graft gene expression profiling (identification of potential ‘tolerance biomarker’ genes) might aid patient selection for early immunosuppressive drug withdrawal. Identification of non-invasive biomarkers is a priority.
-
Early-phase clinical trials of adoptive regulatory immune cell therapy in liver transplantation are underway. In combination with biomarkers, this approach might enable minimization or complete withdrawal of immunosuppression.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Calne, R. Y. et al. Induction of immunological tolerance by porcine liver allografts. Nature 223, 472–476 (1969).
Kamada, N., Davies, H. S. & Roser, B. Reversal of transplantation immunity by liver grafting. Nature 292, 840–842 (1981).
Kamada, N. & Wight, D. G. Antigen-specific immunosuppression induced by liver transplantation in the rat. Transplantation 38, 217–221 (1984).
Navarro, V., Herrine, S., Katopes, C., Colombe, B. & Spain, C. V. The effect of HLA class I (A and B) and class II (DR) compatibility on liver transplantation outcomes: an analysis of the OPTN database. Liver Transpl. 12, 652–658 (2006).
Jakab, S. S. et al. Human leukocyte antigen and adult living-donor liver transplantation outcomes: an analysis of the organ procurement and transplantation network database. Liver Transpl. 13, 1405–1413 (2007).
Starzl, T. E. et al. Homotransplantation of the liver in humans. Surg. Gynecol. Obstet. 117, 659–676 (1963).
Starzl, T. E. et al. Evolution of liver transplantation. Hepatology 2, 614–636 (1982).
White, D. J. G. (Ed.) Cyclosporin A: Proc. International Conference on Cyclosporin A (Elsevier Biomedical, Amsterdam, 1982).
Starzl, T. E., Klintmalm, G. B., Porter, K. A., Iwatsuki, S. & Schroter, G. P. Liver transplantation with use of cyclosporin a and prednisone. N. Engl. J. Med. 305, 266–269 (1981).
Starzl, T. E., Demetris, A. J. & Van Thiel, D. Liver transplantation (1). N. Engl. J. Med. 321, 1014–1022 (1989). This paper is an informative review (part one of two) of the factors influencing the development of clinical liver transplantation from an experimental to a service procedure.
Lemon, K., Al-Khafaji, A. & Humar, A. Critical care management of living donor liver transplants. Crit. Care Clin. 35, 107–116 (2019).
Qian, S. G., Fung, J. J., Demetris, A. V., Ildstad, S. T. & Starzl, T. E. Orthotopic liver transplantation in the mouse. Transplantation 52, 562–564 (1991).
Yokota, S. et al. Orthotopic mouse liver transplantation to study liver biology and allograft tolerance. Nat. Protoc. 11, 1163–1174 (2016).
Qian, S. et al. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology 19, 916–924 (1994). This paper presents an account of liver transplant tolerance across MHC barriers in mice without immunosuppressive therapy, and of donor-derived leukocyte migration to host lymphoid organs.
Zhai, Y., Petrowsky, H., Hong, J. C., Busuttil, R. W. & Kupiec-Weglinski, J. W. Ischaemia–reperfusion injury in liver transplantation — from bench to bedside. Nat. Rev. Gastroenterol. Hepatol. 10, 79–89 (2013).
Lu, L. et al. Innate immune regulations and liver ischemia–reperfusion injury. Transplantation 100, 2601–2610 (2016).
Konishi, T. & Lentsch, A. B. Hepatic ischemia/reperfusion: mechanisms of tissue injury, repair, and regeneration. Gene Expr. 17, 277–287 (2017).
Geissler, E. K. & Schlitt, H. J. Immunosuppression for liver transplantation. Gut 58, 452–463 (2009).
Rodriguez-Peralvarez, M., De la Mata, M. & Burroughs, A. K. Liver transplantation: immunosuppression and oncology. Curr. Opin. Organ. Transpl. 19, 253–260 (2014).
Londono, M. C., Rimola, A., O’Grady, J. & Sanchez-Fueyo, A. Immunosuppression minimization vs. complete drug withdrawal in liver transplantation. J. Hepatol. 59, 872–879 (2013).
Shaked, A. et al. Outcomes of immunosuppression minimization and withdrawal early after liver transplantation. Am. J. Transpl. 19, 1397–1409 (2019).
Vionnet, J. & Sanchez-Fueyo, A. Biomarkers of immune tolerance in liver transplantation. Hum. Immunol. 79, 388–394 (2018).
Levitsky, J. & Feng, S. Tolerance in clinical liver transplantation. Hum. Immunol. 79, 283–287 (2018).
Taner, T. et al. Decreased chronic cellular and antibody-mediated injury in the kidney following simultaneous liver–kidney transplantation. Kidney Int. 89, 909–917 (2016).
Wong, T. W. et al. Liver allograft provides immunoprotection for the cardiac allograft in combined heart–liver transplantation. Am. J. Transpl. 16, 3522–3531 (2016).
Banff Working Group on Liver Allograft Pathology. Importance of liver biopsy findings in immunosuppression management: biopsy monitoring and working criteria for patients with operational tolerance. Liver Transpl. 18, 1154–1170 (2012).
Bohne, F. et al. Intra-graft expression of genes involved in iron homeostasis predicts the development of operational tolerance in human liver transplantation. J. Clin. Invest. 122, 368–382 (2012). This study is the first to offer a set of biomarkers to conduct immunosuppression withdrawal trials in liver transplantation and to point to a critical role for iron metabolism in the regulation of intra-graft alloimmune responses.
Morelli, A. E. & Thomson, A. W. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat. Rev. Immunol. 7, 610–621 (2007).
Wood, K. J., Bushell, A. & Hester, J. Regulatory immune cells in transplantation. Nat. Rev. Immunol. 12, 417–430 (2012).
Todo, S. et al. A pilot study of operational tolerance with a regulatory T-cell-based cell therapy in living donor liver transplantation. Hepatology 64, 632–643 (2016). This study is the first showing evidence that Treg cell-enriched cell therapy is efficient in inducing tolerance in adult living-donor liver transplant recipients early after the liver transplantation.
Thomson, A. W., Humar, A., Lakkis, F. G. & Metes, D. M. Regulatory dendritic cells for promotion of liver transplant operational tolerance: rationale for a clinical trial and accompanying mechanistic studies. Hum. Immunol. 79, 314–321 (2018).
Crispe, I. N. Hepatic T cells and liver tolerance. Nat. Rev. Immunol. 3, 51–62 (2003).
Bismuth, H. Surgical anatomy and anatomical surgery of the liver. World J. Surg. 6, 3–9 (1982).
Thomson, A. W. & Knolle, P. A. Antigen-presenting cell function in the tolerogenic liver environment. Nat. Rev. Immunol. 10, 753–766 (2010).
Doherty, D. G. Immunity, tolerance and autoimmunity in the liver: a comprehensive review. J. Autoimmun. 66, 60–75 (2016).
Holz, L. E., Warren, A., Le Couteur, D. G., Bowen, D. G. & Bertolino, P. CD8+ T cell tolerance following antigen recognition on hepatocytes. J. Autoimmun. 34, 15–22 (2010).
Heymann, F. & Tacke, F. Immunology in the liver — from homeostasis to disease. Nat. Rev. Gastroenterol. Hepatol. 13, 88–110 (2016).
Banchereau, J. & Steinman, R. M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998).
Biswas, S. K. & Lopez-Collazo, E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 30, 475–487 (2009).
Scott, M. J., Liu, S., Shapiro, R. A., Vodovotz, Y. & Billiar, T. R. Endotoxin uptake in mouse liver is blocked by endotoxin pretreatment through a suppressor of cytokine signaling-1-dependent mechanism. Hepatology 49, 1695–1708 (2009).
Fujimoto, M. & Naka, T. SOCS1, a negative regulator of cytokine signals and TLR responses, in human liver diseases. Gastroenterol. Res. Pract. 2010, 470468 (2010).
Xia, S. et al. Hepatic microenvironment programs hematopoietic progenitor differentiation into regulatory dendritic cells, maintaining liver tolerance. Blood 112, 3175–3185 (2008). This paper presents evidence that the liver microenvironment can programme progenitors to differentiate into regulatory dendritic cells in situ and that these cells contribute to the maintenance of liver tolerance.
Zahorchak, A. F. et al. High PD-L1/CD86 MFI ratio and IL-10 secretion characterize human regulatory dendritic cells generated for clinical testing in organ transplantation. Cell Immunol. 323, 9–18 (2018).
Rutella, S. et al. Hepatocyte growth factor favors monocyte differentiation into regulatory interleukin (IL)-10++IL-12low/neg accessory cells with dendritic-cell features. Blood 108, 218–227 (2006).
Cabillic, F. et al. Hepatic environment elicits monocyte differentiation into a dendritic cell subset directing TH2 response. J. Hepatol. 44, 552–559 (2006).
Krueger, P. D., Kim, T. S., Sung, S. S., Braciale, T. J. & Hahn, Y. S. Liver-resident CD103+ dendritic cells prime antiviral CD8+ T cells in situ. J. Immunol. 194, 3213–3222 (2015).
Castellaneta, A., Sumpter, T. L., Chen, L., Tokita, D. & Thomson, A. W. NOD2 ligation subverts IFN-α production by liver plasmacytoid dendritic cells and inhibits their T cell allostimulatory activity via B7-H1 up-regulation. J. Immunol. 183, 6922–6932 (2009).
Tokita, D. et al. Poor allostimulatory function of liver plasmacytoid DC is associated with pro-apoptotic activity, dependent on regulatory T cells. J. Hepatol. 49, 1008–1018 (2008).
Abe, M., Zahorchak, A. F., Colvin, B. L. & Thomson, A. W. Migratory responses of murine hepatic myeloid, lymphoid-related, and plasmacytoid dendritic cells to CC chemokines. Transplantation 78, 762–765 (2004).
O’Connell, P. J. et al. Type-1 polarized nature of mouse liver CD8α– and CD8α+ dendritic cells: tissue-dependent differences offset CD8α-related dendritic cell heterogeneity. Eur. J. Immunol. 33, 2007–2013 (2003).
Lian, Z. X. et al. Heterogeneity of dendritic cells in the mouse liver: identification and characterization of four distinct populations. J. Immunol. 170, 2323–2330 (2003).
Kelly, A. et al. CD141+ myeloid dendritic cells are enriched in healthy human liver. J. Hepatol. 60, 135–142 (2014).
Thomson, A. W. & Lu, L. Are dendritic cells the key to liver transplant tolerance? Immunol. Today 20, 27–32 (1999).
Bamboat, Z. M. et al. Human liver dendritic cells promote T cell hyporesponsiveness. J. Immunol. 182, 1901–1911 (2009).
Abe, M., Tokita, D., Raimondi, G. & Thomson, A. W. Endotoxin modulates the capacity of CpG-activated liver myeloid DC to direct TH1-type responses. Eur. J. Immunol. 36, 2483–2493 (2006).
De Creus, A. et al. Low TLR4 expression by liver dendritic cells correlates with reduced capacity to activate allogeneic T cells in response to endotoxin. J. Immunol. 174, 2037–2045 (2005).
Soysa, R., Wu, X. & Crispe, I. N. Dendritic cells in hepatitis and liver transplantation. Liver Transpl. 23, 1433–1439 (2017).
Sumpter, T. L. et al. DAP12 promotes IRAK-M expression and IL-10 production by liver myeloid dendritic cells and restrains their T cell allostimulatory ability. J. Immunol. 186, 1970–1980 (2011).
Yoshida, O. et al. DAP12 deficiency in liver allografts results in enhanced donor DC migration, augmented effector T cell responses and abrogation of transplant tolerance. Am. J. Transpl. 14, 1791–1805 (2014).
Nakao, T. et al. DNAX activating protein of 12 kDa/triggering receptor expressed on myeloid cells 2 expression by mouse and human liver dendritic cells: functional implications and regulation of liver ischemia–reperfusion injury. Hepatology 70, 696–710 (2019).
Kober, D. L. & Brett, T. J. TREM2–ligand interactions in health and disease. J. Mol. Biol. 429, 1607–1629 (2017).
Steinbrink, K., Wolfl, M., Jonuleit, H., Knop, J. & Enk, A. H. Induction of tolerance by IL-10-treated dendritic cells. J. Immunol. 159, 4772–4780 (1997).
Yamaguchi, Y., Tsumura, H., Miwa, M. & Inaba, K. Contrasting effects of TGF-β1 and TNF-α on the development of dendritic cells from progenitors in mouse bone marrow. Stem Cell 15, 144–153 (1997).
Henning, J. R. et al. Dendritic cells limit fibroinflammatory injury in nonalcoholic steatohepatitis in mice. Hepatology 58, 589–602 (2013).
Jiao, J. et al. Dendritic cell regulation of carbon tetrachloride-induced murine liver fibrosis regression. Hepatology 55, 244–255 (2012).
Bamboat, Z. M. et al. Conventional DCs reduce liver ischemia/reperfusion injury in mice via IL-10 secretion. J. Clin. Invest. 120, 559–569 (2010).
Yoshida, O. et al. CD39 expression by hepatic myeloid dendritic cells attenuates inflammation in liver transplant ischemia–reperfusion injury in mice. Hepatology 58, 2163–2175 (2013).
Khanna, A. et al. Effects of liver-derived dendritic cell progenitors on TH1- and TH2-like cytokine responses in vitro and in vivo. J. Immunol. 164, 1346–1354 (2000).
Rastellini, C. et al. Granulocyte/macrophage colony-stimulating factor-stimulated hepatic dendritic cell progenitors prolong pancreatic islet allograft survival. Transplantation 60, 1366–1370 (1995).
Yokota, S., Yoshida, O., Ono, Y., Geller, D. A. & Thomson, A. W. Liver transplantation in the mouse: insights into liver immunobiology, tissue injury, and allograft tolerance. Liver Transpl. 22, 536–546 (2016).
Kingham, T. P. et al. Murine liver plasmacytoid dendritic cells become potent immunostimulatory cells after Flt-3 ligand expansion. Hepatology 45, 445–454 (2007).
Pillarisetty, V. G., Shah, A. B., Miller, G., Bleier, J. I. & DeMatteo, R. P. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. J. Immunol. 172, 1009–1017 (2004).
Goubier, A. et al. Plasmacytoid dendritic cells mediate oral tolerance. Immunity 29, 464–475 (2008).
Bjorck, P., Coates, P. T., Wang, Z., Duncan, F. J. & Thomson, A. W. Promotion of long-term heart allograft survival by combination of mobilized donor plasmacytoid dendritic cells and anti-CD154 monoclonal antibody. J. Heart Lung Transpl. 24, 1118–1120 (2005).
Abe, M., Wang, Z., de Creus, A. & Thomson, A. W. Plasmacytoid dendritic cell precursors induce allogeneic T-cell hyporesponsiveness and prolong heart graft survival. Am. J. Transpl. 5, 1808–1819 (2005).
David, B. A. et al. Combination of mass cytometry and imaging analysis reveals origin, location, and functional repopulation of liver myeloid cells in mice. Gastroenterology 151, 1176–1191 (2016).
Krenkel, O. & Tacke, F. Liver macrophages in tissue homeostasis and disease. Nat. Rev. Immunol. 17, 306–321 (2017).
MacParland, S. A. et al. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat. Commun. 9, 4383 (2018). This paper presents single-cell resolution of the characteristics of liver-resident cells, providing a map of the human hepatic immune microenvironment.
Shi, J., Fujieda, H., Kokubo, Y. & Wake, K. Apoptosis of neutrophils and their elimination by Kupffer cells in rat liver. Hepatology 24, 1256–1263 (1996).
Meijer, C. et al. Kupffer cell depletion by CI2MDP-liposomes alters hepatic cytokine expression and delays liver regeneration after partial hepatectomy. Liver 20, 66–77 (2000).
Zhu, R. et al. Kupffer cell depletion by gadolinium chloride aggravates liver injury after brain death in rats. Mol. Med. Rep. 17, 6357–6362 (2018).
Roland, C. R., Mangino, M. J., Duffy, B. F. & Flye, M. W. Lymphocyte suppression by Kupffer cells prevents portal venous tolerance induction: a study of macrophage function after intravenous gadolinium. Transplantation 55, 1151–1158 (1993).
Wu, Y. et al. Gadolinium chloride suppresses acute rejection and induces tolerance following rat liver transplantation by inhibiting Kupffer-cell activation. Exp. Ther. Med. 8, 1777–1782 (2014).
You, Q., Cheng, L., Kedl, R. M. & Ju, C. Mechanism of T cell tolerance induction by murine hepatic Kupffer cells. Hepatology 48, 978–990 (2008).
Breous, E., Somanathan, S., Vandenberghe, L. H. & Wilson, J. M. Hepatic regulatory T cells and Kupffer cells are crucial mediators of systemic T cell tolerance to antigens targeting murine liver. Hepatology 50, 612–621 (2009).
Baratelli, F. et al. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J. Immunol. 175, 1483–1490 (2005).
Shimizu, H. et al. Role of Kupffer cells in tolerance induction after portal venous administration of alloantigen. Hepatogastroenterology 56, 783–787 (2009).
Roland, C. R., Walp, L., Stack, R. M. & Flye, M. W. Outcome of Kupffer cell antigen presentation to a cloned murine Th1 lymphocyte depends on the inducibility of nitric oxide synthase by IFN-γ. J. Immunol. 153, 5453–5464 (1994).
Hidaka, M. et al. The Kupffer cell number affects the outcome of living donor liver transplantation from elderly donors. Transpl. Direct 2, e94 (2016).
Meyer, D. et al. Donor-derived alloantigen-presenting cells persist in the liver allograft during tolerance induction. Transpl. Int. 13, 12–20 (2000).
Ebbo, M., Crinier, A., Vely, F. & Vivier, E. Innate lymphoid cells: major players in inflammatory diseases. Nat. Rev. Immunol. 17, 665–678 (2017).
Krueger, P. D. et al. Murine liver-resident group 1 innate lymphoid cells regulate optimal priming of anti-viral CD8+ T cells. J. Leukoc. Biol. 101, 329–338 (2017).
Forkel, M. et al. Composition and functionality of the intrahepatic innate lymphoid cell-compartment in human nonfibrotic and fibrotic livers. Eur. J. Immunol. 47, 1280–1294 (2017).
Shi, F. D., Ljunggren, H. G., La Cava, A. & Van Kaer, L. Organ-specific features of natural killer cells. Nat. Rev. Immunol. 11, 658–671 (2011).
Wu, X., Chen, Y., Wei, H., Sun, R. & Tian, Z. Development of murine hepatic NK cells during ontogeny: comparison with spleen NK cells. Clin. Dev. Immunol. 2012, 759765 (2012).
Lassen, M. G., Lukens, J. R., Dolina, J. S., Brown, M. G. & Hahn, Y. S. Intrahepatic IL-10 maintains NKG2A+Ly49– liver NK cells in a functionally hyporesponsive state. J. Immunol. 184, 2693–2701 (2010).
Shetty, S., Lalor, P. F. & Adams, D. H. Liver sinusoidal endothelial cells—gatekeepers of hepatic immunity. Nat. Rev. Gastroenterol. Hepatol. 15, 555–567 (2018).
Lohse, A. W. et al. Antigen-presenting function and B7 expression of murine sinusoidal endothelial cells and Kupffer cells. Gastroenterology 110, 1175–1181 (1996).
Leifeld, L. et al. Enhanced expression of CD80 (B7-1), CD86 (B7-2), and CD40 and their ligands CD28 and CD154 in fulminant hepatic failure. Am. J. Pathol. 154, 1711–1720 (1999).
Knolle, P. A. et al. Induction of cytokine production in naive CD4+ T cells by antigen-presenting murine liver sinusoidal endothelial cells but failure to induce differentiation toward TH1 cells. Gastroenterology 116, 1428–1440 (1999).
Limmer, A. et al. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat. Med. 6, 1348–1354 (2000). This paper presents evidence that the outcome of antigen (cross-)presentation by liver sinusoidal lining cells is CD8+ T cell tolerance rather than immunity.
Ge, X., Nowak, G., Ericzon, B. G. & Sumitran-Holgersson, S. Liver sinusoidal endothelial cell function in rejected and spontaneously accepted rat liver allografts. Transpl. Int. 21, 49–56 (2008).
Tang, L. et al. Liver sinusoidal endothelial cell lectin, LSECtin, negatively regulates hepatic T-cell immune response. Gastroenterology 137, 1498–1508.e1–e5 (2009).
Kruse, N. et al. Priming of CD4+ T cells by liver sinusoidal endothelial cells induces CD25low forkhead box protein 3-regulatory T cells suppressing autoimmune hepatitis. Hepatology 50, 1904–1913 (2009).
Schildberg, F. A. et al. Liver sinusoidal endothelial cells veto CD8 T cell activation by antigen-presenting dendritic cells. Eur. J. Immunol. 38, 957–967 (2008).
Kern, M. et al. Virally infected mouse liver endothelial cells trigger CD8+ T-cell immunity. Gastroenterology 138, 336–346 (2010).
Gandhi, C. R. in Molecular Pathology Library Part 1 (ed. Monga, S. P.) 53-80 (Springer, 2010).
Yu, M. C. et al. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology 40, 1312–1321 (2004).
Yang, H. R. et al. A critical role of TRAIL expressed on cotransplanted hepatic stellate cells in prevention of islet allograft rejection. Microsurgery 30, 332–337 (2010).
Jiang, G. et al. Hepatic stellate cells preferentially expand allogeneic CD4+CD25+FoxP3+ regulatory T cells in an IL-2-dependent manner. Transplantation 86, 1492–1502 (2008).
Sumpter, T. L. et al. Hepatic stellate cells undermine the allostimulatory function of liver myeloid dendritic cells via STAT3-dependent induction of IDO. J. Immunol. 189, 3848–3858 (2012).
Dangi, A. et al. Selective expansion of allogeneic regulatory T cells by hepatic stellate cells: role of endotoxin and implications for allograft tolerance. J. Immunol. 188, 3667–3677 (2012). This paper presents data suggesting that endotoxin-stimulated HSCs might serve as important immune regulators in liver transplantation by inducing selective expansion of tolerance-promoting Treg cells and reducing inflammation and alloimmunity.
Schildberg, F. A. et al. Murine hepatic stellate cells veto CD8 T cell activation by a CD54-dependent mechanism. Hepatology 54, 262–272 (2011).
Kumar, S., Wang, J., Thomson, A. W. & Gandhi, C. R. Hepatic stellate cells increase the immunosuppressive function of natural Foxp3+ regulatory T cells via IDO-induced AhR activation. J. Leukoc. Biol. 101, 429–438 (2017).
Li, Y., Lu, L., Qian, S., Fung, J. J. & Lin, F. Hepatic stellate cells directly inhibit B cells via programmed death-ligand 1. J. Immunol. 196, 1617–1625 (2016).
Gabrilovich, D. I. & Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174 (2009).
Hochst, B. et al. Activated human hepatic stellate cells induce myeloid derived suppressor cells from peripheral blood monocytes in a CD44-dependent fashion. J. Hepatol. 59, 528–535 (2013).
Norris, S. et al. Resident human hepatic lymphocytes are phenotypically different from circulating lymphocytes. J. Hepatol. 28, 84–90 (1998).
Guidotti, L. G. et al. Immunosurveillance of the liver by intravascular effector CD8+ T cells. Cell 161, 486–500 (2015).
Pallett, L. J. et al. IL-2high tissue-resident T cells in the human liver: sentinels for hepatotropic infection. J. Exp. Med. 214, 1567–1580 (2017).
Bertolino, P., Trescol-Biemont, M. C. & Rabourdin-Combe, C. Hepatocytes induce functional activation of naive CD8+ T lymphocytes but fail to promote survival. Eur. J. Immunol. 28, 221–236 (1998).
Morimoto, J., Tan, X., Teague, R. M., Ohlen, C. & Greenberg, P. D. Induction of tolerance in CD8+ T cells to a transgenic autoantigen expressed in the liver does not require cross-presentation. J. Immunol. 178, 6849–6860 (2007).
Zierden, M., Kuhnen, E., Odenthal, M. & Dienes, H. P. Effects and regulation of autoreactive CD8+ T cells in a transgenic mouse model of autoimmune hepatitis. Gastroenterology 139, 975–986.e1–e3 (2010).
Klein, I. & Crispe, I. N. Complete differentiation of CD8+ T cells activated locally within the transplanted liver. J. Exp. Med. 203, 437–447 (2006).
Wuensch, S. A., Pierce, R. H. & Crispe, I. N. Local intrahepatic CD8+ T cell activation by a non-self-antigen results in full functional differentiation. J. Immunol. 177, 1689–1697 (2006).
Tay, S. S. et al. Antigen expression level threshold tunes the fate of CD8 T cells during primary hepatic immune responses. Proc. Natl Acad. Sci. USA 111, E2540–E2549 (2014). This study reveals a hierarchy of factors that determine the fate of CD8+ T cells during hepatic immune responses and provides an explanation for different immune outcomes under various immune-mediated liver pathological conditions.
Bowen, D. G. et al. The site of primary T cell activation is a determinant of the balance between intrahepatic tolerance and immunity. J. Clin. Invest. 114, 701–712 (2004). This paper presents evidence that whereas naive CD8+ T cells activated in lymph nodes mediate hepatitis, those that undergo primary activation in the liver are defective in cytotoxic function, with a shortened half-life and inability to mediate hepatocellular injury.
Holz, L. E. et al. Naive CD8 T cell activation by liver bone marrow-derived cells leads to a “neglected” IL-2lowBimhigh phenotype, poor CTL function and cell death. J. Hepatol. 57, 830–836 (2012).
Bertolino, P. et al. Death by neglect as a deletional mechanism of peripheral tolerance. Int. Immunol. 11, 1225–1238 (1999).
Qian, S. et al. Apoptosis within spontaneously accepted mouse liver allografts: evidence for deletion of cytotoxic T cells and implications for tolerance induction. J. Immunol. 158, 4654–4661 (1997). This paper presents observations suggesting that deletion of graft-infiltrating cytotoxic T cells might be responsible for spontaneous liver allograft acceptance in the mouse.
Meyer, D. et al. Apoptosis of alloreactive T cells in liver allografts during tolerance induction. Transpl. Proc. 31, 474 (1999).
Holz, L. E. et al. Intrahepatic murine CD8 T-cell activation associates with a distinct phenotype leading to Bim-dependent death. Gastroenterology 135, 989–997 (2008).
Sharland, A. et al. Evidence that apoptosis of activated T cells occurs in spontaneous tolerance of liver allografts and is blocked by manipulations which break tolerance. Transplantation 68, 1736–1745 (1999).
Subleski, J. J. et al. TCR-dependent and -independent activation underlie liver-specific regulation of NKT cells. J. Immunol. 186, 838–847 (2011).
Farci, P. et al. Lack of protective immunity against reinfection with hepatitis C virus. Science 258, 135–140 (1992).
Chisari, F. V. & Ferrari, C. Hepatitis B virus immunopathogenesis. Annu. Rev. Immunol. 13, 29–60 (1995).
Luth, S. et al. Ectopic expression of neural autoantigen in mouse liver suppresses experimental autoimmune neuroinflammation by inducing antigen-specific Tregs. J. Clin. Invest. 118, 3403–3410 (2008).
Akbarpour, M. et al. Insulin B chain 9-23 gene transfer to hepatocytes protects from type 1 diabetes by inducing Ag-specific FoxP3+ Tregs. Sci. Transl Med. 7, 289ra281 (2015).
Paul-Heng, M. et al. Direct recognition of hepatocyte-expressed MHC class I alloantigens is required for tolerance induction. JCI Insight https://doi.org/10.1172/jci.insight.97500 (2018).
Kamada, N., Brons, G. & Davies, H. S. Fully allogeneic liver grafting in rats induces a state of systemic nonreactivity to donor transplantation antigens. Transplantation 29, 429–431 (1980).
Kamada, N., Davies, H. S., Wight, D., Culank, L. & Roser, B. Liver transplantation in the rat. Biochemical and histological evidence of complete tolerance induction in non-rejector strains. Transplantation 35, 304–311 (1983).
Kamada, N. The immunology of experimental liver transplantation in the rat. Immunology 55, 369–389 (1985).
Murase, N. et al. Suppression of allograft rejection with FK506. I. Prolonged cardiac and liver survival in rats following short-course therapy. Transplantation 50, 186–189 (1990).
Vitalone, M. J. et al. Liver microRNA profile of induced allograft tolerance. Transplantation 100, 781–790 (2016).
Kobayashi, E. et al. Prevention by liver transplantation of the graft-versus-host reaction and allograft rejection in a rat model of small bowel transplantation. Transplantation 57, 177–181 (1994).
Sarnacki, S. et al. Decreased expression of the interleukin 2 receptor on CD8 recipient lymphocytes in intestinal grafts rendered tolerant by liver transplantation in rats. Gut 43, 849–855 (1998).
Murase, N. et al. Multilineage hematopoietic reconstitution of supralethally irradiated rats by syngeneic whole organ transplantation. With oarticular reference to the liver. Transplantation 61, 1–4 (1996).
Lu, L. et al. Growth of donor-derived dendritic cells from the bone marrow of murine liver allograft recipients in response to granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 182, 379–387 (1995).
Wang, X. Q. et al. Hematopoietic chimerism in liver transplantation patients and hematopoietic stem/progenitor cells in adult human liver. Hepatology 56, 1557–1566 (2012).
Shimizu, Y. et al. Restoration of tolerance to rat hepatic allografts by spleen-derived passenger leukocytes. Transpl. Int. 9, 593–595 (1996).
Xie, Y. et al. Delayed donor bone marrow infusion induces liver transplant tolerance. Transplantation 101, 1056–1066 (2017).
Tashiro, H. et al. Assessment of microchimerism in rat liver transplantation by polymerase chain reaction. Hepatology 23, 828–834 (1996).
Demetris, A. J., Murase, N. & Starzl, T. E. Donor dendritic cells after liver and heart allotransplantation under short-term immunosuppression. Lancet 339, 1610 (1992).
Yoshimura, S., Gotoh, S. & Kamada, N. Immunological tolerance induced by liver grafting in the rat: splenic macrophages and T cells mediate distinct phases of immunosuppressive activity. Clin. Exp. Immunol. 85, 121–127 (1991).
Sriwatanawongsa, V., Davies, H. S. & Calne, R. Y. The essential roles of parenchymal tissues and passenger leukocytes in the tolerance induced by liver grafting in rats. Nat. Med. 1, 428–432 (1995).
Sun, J., McCaughan, G. W., Gallagher, N. D., Sheil, A. G. & Bishop, G. A. Deletion of spontaneous rat liver allograft acceptance by donor irradiation. Transplantation 60, 233–236 (1995).
Sun, J. et al. Tolerance to rat liver allografts: IV. Acceptance depends on the quantity of donor tissue and on donor leukocytes. Transplantation 62, 1725–1730 (1996).
Bishop, G. A. et al. Tolerance to rat liver allografts. III. Donor cell migration and tolerance-associated cytokine production in peripheral lymphoid tissues. J. Immunol. 156, 4925–4931 (1996).
Kreisel, D. et al. The role of passenger leukocyte genotype in rejection and acceptance of rat liver allografts. Transplantation 73, 1501–1507 (2002).
Sharland, A. et al. Kinetics of intragraft cytokine expression, cellular infiltration, and cell death in rejection of renal allografts compared with acceptance of liver allografts in a rat model: early activation and apoptosis is associated with liver graft acceptance. Transplantation 65, 1370–1377 (1998).
Dresske, B., Lin, X., Huang, D. S., Zhou, X. & Fandrich, F. Spontaneous tolerance: experience with the rat liver transplant model. Hum. Immunol. 63, 853–861 (2002).
Bishop, G. A., Wang, C., Sharland, A. F. & McCaughan, G. Spontaneous acceptance of liver transplants in rodents: evidence that liver leucocytes induce recipient T-cell death by neglect. Immunol. Cell Biol. 80, 93–100 (2002).
Benseler, V., Tay, S. S., Bowen, D. G. & Bertolino, P. Role of the hepatic parenchyma in liver transplant tolerance: a paradigm revisited. Dig. Dis. 29, 391–401 (2011).
Tay, S. S. et al. Differential migration of passenger leukocytes and rapid deletion of naive alloreactive CD8 T cells after mouse liver transplantation. Liver Transpl. 19, 1224–1235 (2013).
Ring, G. H. et al. Interferon-γ is necessary for initiating the acute rejection of major histocompatibility complex class II-disparate skin allografts. Transplantation 67, 1362–1365 (1999).
Mele, T. S. et al. IFN-γ is an absolute requirement for spontaneous acceptance of liver allografts. Am. J. Transpl. 3, 942–951 (2003).
Morita, M. et al. Rejection triggers liver transplant tolerance: involvement of mesenchyme-mediated immune control mechanisms in mice. Hepatology 62, 915–931 (2015).
Morita, M. et al. Identification of microRNAs involved in acute rejection and spontaneous tolerance in murine hepatic allografts. Sci. Rep. 4, 6649 (2014).
Li, W., Zheng, X. X., Kuhr, C. S. & Perkins, J. D. CTLA4 engagement is required for induction of murine liver transplant spontaneous tolerance. Am. J. Transpl. 5, 978–986 (2005).
Ye, Y. et al. Galectin-1 prolongs survival of mouse liver allografts from Flt3L-pretreated donors. Am. J. Transpl. 13, 569–579 (2013).
Jiang, Z. J. et al. Galectin-1 restores immune tolerance to liver transplantation through activation of hepatic stellate cells. Cell Physiol. Biochem. 48, 863–879 (2018).
Li, W. et al. New insights into mechanisms of spontaneous liver transplant tolerance: the role of Foxp3-expressing CD25+CD4+ regulatory T cells. Am. J. Transpl. 8, 1639–1651 (2008). This paper presents evidence that Treg cells have an important role in spontaneous acceptance of liver allografts in mice.
Jiang, X. et al. The importance of CD25+CD4+ regulatory T cells in mouse hepatic allograft tolerance. Liver Transpl. 12, 1112–1118 (2006).
Demirkiran, A. et al. Allosuppressive donor CD4+CD25+ regulatory T cells detach from the graft and circulate in recipients after liver transplantation. J. Immunol. 178, 6066–6072 (2007).
Ono, Y. et al. Graft-infiltrating PD-L1hi cross-dressed dendritic cells regulate antidonor T cell responses in mouse liver transplant tolerance. Hepatology 67, 1499–1515 (2018). This study is the first demonstration that cross-dressing (acquisition of intact donor MHC antigen) of host dendritic cells has an important role in experimental organ transplant tolerance.
van der Touw, W. & Bromberg, J. S. Natural killer cells and the immune response in solid organ transplantation. Am. J. Transpl. 10, 1354–1358 (2010).
Moroso, V. et al. Liver grafts contain a unique subset of natural killer cells that are transferred into the recipient after liver transplantation. Liver Transpl. 16, 895–908 (2010).
Harmon, C., Sanchez-Fueyo, A., O’Farrelly, C. & Houlihan, D. D. Natural killer cells and liver transplantation: orchestrators of rejection or tolerance? Am. J. Transpl. 16, 751–757 (2016).
Fahrner, R., Dondorf, F., Ardelt, M., Settmacher, U. & Rauchfuss, F. Role of NK, NKT cells and macrophages in liver transplantation. World J. Gastroenterol. 22, 6135–6144 (2016).
Martinez-Llordella, M. et al. Using transcriptional profiling to develop a diagnostic test of operational tolerance in liver transplant recipients. J. Clin. Invest. 118, 2845–2857 (2008). This work reveals the first data suggesting that transcriptional profiling of peripheral blood can be employed to identify liver transplant recipients who can discontinue immunosuppressive therapy.
Obara, H. et al. IFN-γ, produced by NK cells that infiltrate liver allografts early after transplantation, links the innate and adaptive immune responses. Am. J. Transpl. 5, 2094–2103 (2005).
Farges, O., Morris, P. J. & Dallman, M. J. Spontaneous acceptance of rat liver allografts is associated with an early downregulation of intragraft interleukin-4 messenger RNA expression. Hepatology 21, 767–775 (1995).
Wang, C. et al. Posttransplant interleukin-4 treatment converts rat liver allograft tolerance to rejection. Transplantation 79, 1116–1120 (2005).
Xie, L. et al. Identification of a novel biomarker gene set with sensitivity and specificity for distinguishing between allograft rejection and tolerance. Liver Transpl. 18, 444–454 (2012).
Sanchez-Fueyo, A., Weber, M., Domenig, C., Strom, T. B. & Zheng, X. X. Tracking the immunoregulatory mechanisms active during allograft tolerance. J. Immunol. 168, 2274–2281 (2002).
Sandner, S. E. et al. Role of the programmed death-1 pathway in regulation of alloimmune responses in vivo. J. Immunol. 174, 3408–3415 (2005).
Taubert, R. et al. Hepatic infiltrates in operational tolerant patients after liver transplantation show enrichment of regulatory T cells before proinflammatory genes are downregulated. Am. J. Transpl. 16, 1285–1293 (2016).
Bohne, F. et al. HCV-induced immune responses influence the development of operational tolerance after liver transplantation in humans. Sci. Transl Med. 6, 242ra281 (2014).
Starzl, T. E., Demetris, A. J. & Van Thiel, D. Liver transplantation (2). N. Engl. J. Med. 321, 1092–1099 (1989).
US Multicenter FK506 Liver Study Group. A comparison of tacrolimus (FK 506) and cyclosporine for immunosuppression in liver transplantation. N. Engl. J. Med. 331, 1110–1115 (1994).
Moini, M., Schilsky, M. L. & Tichy, E. M. Review on immunosuppression in liver transplantation. World J. Hepatol. 7, 1355–1368 (2015).
Rodriguez-Peralvarez, M. et al. Lack of agreement for defining ‘clinical suspicion of rejection’ in liver transplantation: a model to select candidates for liver biopsy. Transpl. Int. 28, 455–464 (2015).
Choudhary, N. S. et al. Acute and chronic rejection after liver transplantation: what a clinician needs to know. J. Clin. Exp. Hepatol. 7, 358–366 (2017).
[No authors listed]. Banff schema for grading liver allograft rejection: an international consensus document. Hepatology 25, 658–663 (1997).
Wiesner, R. H. et al. Acute hepatic allograft rejection: incidence, risk factors, and impact on outcome. Hepatology 28, 638–645 (1998).
Shaked, A. et al. Incidence and severity of acute cellular rejection in recipients undergoing adult living donor or deceased donor liver transplantation. Am. J. Transpl. 9, 301–308 (2009).
Jadlowiec, C. C. et al. Not all cellular rejections are the same: differences in early and late hepatic allograft rejection. Liver Transpl. 25, 425–435 (2019).
Levitsky, J. et al. Acute rejection increases risk of graft failure and death in recent liver transplant recipients. Clin. Gastroenterol. Hepatol. 15, 584–593.e2 (2017).
Demetris, A. et al. Update of the international banff schema for liver allograft rejection: working recommendations for the histopathologic staging and reporting of chronic rejection. An international panel. Hepatology 31, 792–799 (2000).
Evans, H. M., Kelly, D. A., McKiernan, P. J. & Hubscher, S. Progressive histological damage in liver allografts following pediatric liver transplantation. Hepatology 43, 1109–1117 (2006).
Venturi, C. et al. Dynamics of allograft fibrosis in pediatric liver transplantation. Am. J. Transpl. 14, 1648–1656 (2014).
Feng, S. et al. Evidence of chronic allograft injury in liver biopsies from long-term pediatric recipients of liver transplants. Gastroenterology 155, 1838–1851.e7 (2018). This paper reveals that a long-term paediatric liver transplant recipient population with consistently normal liver test results has a high prevalence of chronic injury and that a subset of biopsy samples with interface inflammation express the transcriptional programme of T cell-mediated rejection.
Londono, M. C. et al. Molecular profiling of subclinical inflammatory lesions in long-term surviving adult liver transplant recipients. J. Hepatol. 69, 626–634 (2018).
Balan, V. et al. Long-term outcome of human leukocyte antigen mismatching in liver transplantation: results of the National Institute of Diabetes and Digestive and Kidney Diseases Liver Transplantation Database. Hepatology 48, 878–888 (2008).
Lan, X. et al. Impact of human leukocyte antigen mismatching on outcomes of liver transplantation: a meta-analysis. World J. Gastroenterol. 16, 3457–3464 (2010).
Uchiyama, H. et al. Relevance of HLA compatibility in living donor liver transplantation: the double-edged sword associated with the patient outcome. Clin. Transpl. 26, E522–E529 (2012).
Forner, D., Liwski, R. & Alwayn, I. Human leukocyte antigen, allele, and eplet mismatches in liver transplantation; observations from a small, single center cohort. Hum. Immunol. 79, 154–159 (2018).
O’Leary, J. G. et al. The role of donor-specific HLA alloantibodies in liver transplantation. Am. J. Transpl. 14, 779–787 (2014).
Ruiz, R. et al. Implications of a positive crossmatch in liver transplantation: a 20-year review. Liver Transpl. 18, 455–460 (2012).
Castillo-Rama, M. et al. Preformed antibodies detected by cytotoxic assay or multibead array decrease liver allograft survival: role of human leukocyte antigen compatibility. Liver Transpl. 14, 554–562 (2008).
O’Leary, J. G. et al. Preformed class II donor-specific antibodies are associated with an increased risk of early rejection after liver transplantation. Liver Transpl. 19, 973–980 (2013).
Wozniak, L. J. et al. Donor-specific HLA antibodies are associated with late allograft dysfunction after pediatric liver transplantation. Transplantation 99, 1416–1422 (2015).
O’Leary, J. G. et al. Proposed diagnostic criteria for chronic antibody-mediated rejection in liver allografts. Am. J. Transpl. 16, 603–614 (2016).
Taner, T. et al. Prevalence, course and impact of HLA donor-specific antibodies in liver transplantation in the first year. Am. J. Transpl. 12, 1504–1510 (2012).
Benitez, C. et al. Prospective multicenter clinical trial of immunosuppressive drug withdrawal in stable adult liver transplant recipients. Hepatology 58, 1824–1835 (2013). This paper presents the largest immunosuppression withdrawal trial in adult liver transplantation published to date, paving the way for prospective studies employing clinically applicable diagnostic biomarkers that are currently underway.
Feng, S. et al. Complete immunosuppression withdrawal and subsequent allograft function among pediatric recipients of parental living donor liver transplants. JAMA 307, 283–293 (2012). This work presents a pilot study showing that 60% of paediatric recipients of parental living-donor liver transplants could achieve tolerance, as assessed by normal graft function and stable allograft histology for at least 1 year.
Morath, C., Opelz, G., Zeier, M. & Susal, C. Clinical relevance of HLA antibody monitoring after kidney transplantation. J. Immunol. Res. 2014, 845040 (2014).
Berry, G. J. et al. The 2013 International Society for Heart and Lung Transplantation Working Formulation for the standardization of nomenclature in the pathologic diagnosis of antibody-mediated rejection in heart transplantation. J. Heart Lung Transpl. 32, 1147–1162 (2013).
de Reuver, P. et al. Recipient ctla-4 + 49G/G genotype is associated with reduced incidence of acute rejection after liver transplantation. Am. J. Transpl. 3, 1587–1594 (2003).
Warle, M. C., Metselaar, H. J., Hop, W. C. & Tilanus, H. W. Cytokine gene polymorphisms and acute liver graft rejection: a meta-analysis. Liver Transpl. 11, 19–26 (2005).
Shaked, A. et al. An ectopically expressed serum miRNA signature is prognostic, diagnostic, and biologically related to liver allograft rejection. Hepatology 65, 269–280 (2017).
Bonaccorsi-Riani, E. et al. Molecular characterization of acute cellular rejection occurring during intentional immunosuppression withdrawal in liver transplantation. Am. J. Transpl. 16, 484–496 (2016). This work provides a molecular signature for acute cellular rejection in liver transplantation as well as insights into the molecular processes underlying this process.
Taner, T. Liver transplantation: rejection and tolerance. Liver Transpl. 23, S85–S88 (2017).
Goldschmidt, I. et al. Immune monitoring after pediatric liver transplantation — the prospective ChilSFree cohort study. BMC Gastroenterol. 18, 63 (2018).
Rebollo-Mesa, I. et al. Biomarkers of tolerance in kidney transplantation: are we predicting tolerance or response to immunosuppressive treatment? Am. J. Transpl. 16, 3443–3457 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02498977 (2019).
De Domenico, I. et al. Hepcidin mediates transcriptional changes that modulate acute cytokine-induced inflammatory responses in mice. J. Clin. Invest. 120, 2395–2405 (2010).
Girelli, D. et al. Reduced serum hepcidin levels in patients with chronic hepatitis C. J. Hepatol. 51, 845–852 (2009).
Demirkiran, A. et al. Low circulating regulatory T-cell levels after acute rejection in liver transplantation. Liver Transpl. 12, 277–284 (2006).
Pons, J. A. et al. FoxP3 in peripheral blood is associated with operational tolerance in liver transplant patients during immunosuppression withdrawal. Transplantation 86, 1370–1378 (2008).
Baroja-Mazo, A. et al. Tolerance in liver transplantation: biomarkers and clinical relevance. World J. Gastroenterol. 22, 7676–7691 (2016).
Lau, A. H. et al. Mass cytometry reveals a distinct immunoprofile of operational tolerance in pediatric liver transplantation. Pediatr. Transpl. 20, 1072–1080 (2016).
Donckier, V. et al. Acute liver transplant rejection upon immunosuppression withdrawal in a tolerance induction trial: potential role of IFN-γ-secreting CD8+ T cells. Transplantation 87, S91–S95 (2009).
Wong, T., Nouri-Aria, K. T., Devlin, J., Portmann, B. & Williams, R. Tolerance and latent cellular rejection in long-term liver transplant recipients. Hepatology 28, 443–449 (1998).
Martinez-Llordella, M. et al. Multiparameter immune profiling of operational tolerance in liver transplantation. Am. J. Transpl. 7, 309–319 (2007).
Li, Y. et al. Analyses of peripheral blood mononuclear cells in operational tolerance after pediatric living donor liver transplantation. Am. J. Transpl. 4, 2118–2125 (2004). This study shows for the first time differences in the phenotype of lymphocytes present in peripheral blood mononuclear cells in operationally tolerant patients and age-matched volunteers with normal liver function via flow cytometry.
Mazariegos, G. V. et al. Dendritic cell subset ratio in tolerant, weaning and non-tolerant liver recipients is not affected by extent of immunosuppression. Am. J. Transpl. 5, 314–322 (2005).
Tokita, D. et al. High PD-L1/CD86 ratio on plasmacytoid dendritic cells correlates with elevated T-regulatory cells in liver transplant tolerance. Transplantation 85, 369–377 (2008).
Rogers, N. M., Isenberg, J. S. & Thomson, A. W. Plasmacytoid dendritic cells: no longer an enigma and now key to transplant tolerance? Am. J. Transpl. 13, 1125–1133 (2013).
Morita, M. et al. PD-1/B7-H1 interaction contribute to the spontaneous acceptance of mouse liver allograft. Am. J. Transpl. 10, 40–46 (2010).
De Bruyn, P. et al. Immune checkpoint blockade for organ transplant patients with advanced cancer: how far can we go? Curr. Opin. Oncol. 31, 54–64 (2019).
Venkatachalam, K., Malone, A. F., Heady, B., Delos Santos, R. & Alhamad, T. Poor outcomes with the use of checkpoint inhibitors in kidney transplant recipients. Transplantation https://doi.org/10.1097/TP.0000000000002914 (2019).
Munker, S. & De Toni, E. N. Use of checkpoint inhibitors in liver transplant recipients. United Eur. Gastroenterol. J. 6, 970–973 (2018).
Feng, S. & Bucuvalas, J. Tolerance after liver transplantation: where are we? Liver Transpl. 23, 1601–1614 (2017).
Mastoridis, S., Martinez-Llordella, M. & Sanchez-Fueyo, A. Immunotolerance in liver transplantation. Semin. Liver Dis. 37, 95–108 (2017).
Sanchez-Fueyo, A. Tolerance profiles and immunosuppression. Liver Transpl. 19, S44–S48 (2013).
de la Garza, R. G. et al. Trial of complete weaning from immunosuppression for liver transplant recipients: factors predictive of tolerance. Liver Transpl. 19, 937–944 (2013).
Donckier, V. et al. Expansion of memory-type CD8+ T cells correlates with the failure of early immunosuppression withdrawal after cadaver liver transplantation using high-dose ATG induction and rapamycin. Transplantation 96, 306–315 (2013).
Lerut, J. & Sanchez-Fueyo, A. An appraisal of tolerance in liver transplantation. Am. J. Transpl. 6, 1774–1780 (2006).
Tang, Q. & Vincenti, F. Transplant trials with Tregs: perils and promises. J. Clin. Invest. 127, 2505–2512 (2017).
Thomson, A. W. & Ezzelarab, M. B. Regulatory dendritic cells: profiling, targeting, and therapeutic application. Curr. Opin. Organ. Transpl. 23, 538–545 (2018).
Detry, O. et al. Infusion of mesenchymal stromal cells after deceased liver transplantation: a phase I–II, open-label, clinical study. J. Hepatol. 67, 47–55 (2017).
Soeder, Y. et al. First-in-human case study: multipotent adult progenitor cells for immunomodulation after liver transplantation. Stem Cell Transl Med. 4, 899–904 (2015).
Donckier, V. et al. Early immunosuppression withdrawal after living donor liver transplantation and donor stem cell infusion. Liver Transpl. 12, 1523–1528 (2006).
Hartleif, S. et al. Safety and tolerance of donor-derived mesenchymal stem cells in pediatric living-donor liver transplantation: the MYSTEP1 study. Stem Cell Int. 2017, 2352954 (2017).
Thomson, A. W. et al. Prospective clinical testing of regulatory dendritic cells in organ transplantation. Front. Immunol. 7, 15 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03164265 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03577431 (2019).
Pommey, S. et al. Liver grafts from CD39-overexpressing rodents are protected from ischemia reperfusion injury due to reduced numbers of resident CD4+ T cells. Hepatology 57, 1597–1606 (2013).
Liu, Y. et al. Negative CD4+TIM-3 signaling confers resistance against cold preservation damage in mouse liver transplantation. Am. J. Transpl. 15, 954–964 (2015).
Conzelmann, L. O. et al. Graft tumor necrosis factor receptor-1 protects after mouse liver transplantation whereas host tumor necrosis factor receptor-1 promotes injury. Transplantation 82, 1214–1220 (2006).
Ueki, S. et al. Critical role of interferon regulatory factor-1 in murine liver transplant ischemia reperfusion injury. Hepatology 51, 1692–1701 (2010).
Yokota, S. et al. IRF-1 promotes liver transplant ischemia/reperfusion injury via hepatocyte IL-15/IL-15Rα production. J. Immunol. 194, 6045–6056 (2015).
Xie, J. F. et al. Selective neutralization of the chemokine TCA3 reduces the increased injury of partial versus whole liver transplants induced by cold preservation. Transplantation 82, 1501–1509 (2006).
Shen, X. D. et al. Absence of Toll-like receptor 4 (TLR4) signaling in the donor organ reduces ischemia and reperfusion injury in a murine liver transplantation model. Liver Transpl. 13, 1435–1443 (2007).
Theruvath, T. P. et al. C-Jun N-terminal kinase 2 promotes graft injury via the mitochondrial permeability transition after mouse liver transplantation. Am. J. Transpl. 8, 1819–1828 (2008).
Ueki, S. et al. Hepatic B7 homolog 1 expression is essential for controlling cold ischemia/reperfusion injury after mouse liver transplantation. Hepatology 54, 216–228 (2011).
Zhang, Y. et al. Targeting TIM-1 on CD4 T cells depresses macrophage activation and overcomes ischemia–reperfusion injury in mouse orthotopic liver transplantation. Am. J. Transpl. 13, 56–66 (2013).
Shen, X. D. et al. Disruption of Type-I IFN pathway ameliorates preservation damage in mouse orthotopic liver transplantation via HO-1 dependent mechanism. Am. J. Transpl. 12, 1730–1739 (2012).
Tsung, A. et al. Increasing numbers of hepatic dendritic cells promote HMGB1-mediated ischemia–reperfusion injury. J. Leukoc. Biol. 81, 119–128 (2007).
Dubois, B. et al. Sequential role of plasmacytoid dendritic cells and regulatory T cells in oral tolerance. Gastroenterology 137, 1019–1028 (2009).
Li, W. et al. Il-12 antagonism enhances apoptotic death of T cells within hepatic allografts from Flt3 ligand-treated donors and promotes graft acceptance. J. Immunol. 166, 5619–5628 (2001).
Jomantaite, I. et al. Hepatic dendritic cell subsets in the mouse. Eur. J. Immunol. 34, 355–365 (2004).
Watanabe, T. et al. A liver tolerates a portal antigen by generating CD11c+ cells, which select Fas ligand+ TH2 cells via apoptosis. Hepatology 38, 403–412 (2003).
Bertolino, P., Heath, W. R., Hardy, C. L., Morahan, G. & Miller, J. F. Peripheral deletion of autoreactive CD8+ T cells in transgenic mice expressing H-2Kb in the liver. Eur. J. Immunol. 25, 1932–1942 (1995).
Villadangos, J. A. & Young, L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity 29, 352–361 (2008).
Knolle, P. A. et al. Interleukin-10 expression is autoregulated at the transcriptional level in human and murine Kupffer cells. Hepatology 27, 93–99 (1998).
Bissell, D. M., Wang, S. S., Jarnagin, W. R. & Roll, F. J. Cell-specific expression of transforming growth factor-β in rat liver. Evidence for autocrine regulation of hepatocyte proliferation. J. Clin. Invest. 96, 447–455 (1995).
Schmieg, J., Yang, G., Franck, R. W., Van Rooijen, N. & Tsuji, M. Glycolipid presentation to natural killer T cells differs in an organ-dependent fashion. Proc. Natl Acad. Sci. USA 102, 1127–1132 (2005).
Wiegard, C. et al. Murine liver antigen presenting cells control suppressor activity of CD4+CD25+ regulatory T cells. Hepatology 42, 193–199 (2005).
Kuniyasu, Y., Marfani, S. M., Inayat, I. B., Sheikh, S. Z. & Mehal, W. Z. Kupffer cells required for high affinity peptide-induced deletion, not retention, of activated CD8+ T cells by mouse liver. Hepatology 39, 1017–1027 (2004).
Giannandrea, M., Pierce, R. H. & Crispe, I. N. Indirect action of tumor necrosis factor-α in liver injury during the CD8+ T cell response to an adeno-associated virus vector in mice. Hepatology 49, 2010–2020 (2009).
Thomson, A. W. et al. Identification of donor-derived dendritic cell progenitors in bone marrow of spontaneously tolerant liver allograft recipients. Transplantation 60, 1555–1559 (1995).
Liu, H. et al. PD-L1 signal on liver dendritic cells is critical for Foxp3+CD4+CD25+ Treg and liver tolerance induction in mice. Transpl. Proc. 45, 1853–1855 (2013).
Morita, M. et al. Spontaneous tolerance involving natural killer T cells after hepatic grafting in mice. Transpl. Immunol. 18, 142–145 (2007).
Uchiyama, H. et al. Crucial Fas–Fas ligand interaction in spontaneous acceptance of hepatic allografts in mice. Immunology 105, 450–457 (2002).
Assy, N. et al. Randomized controlled trial of total immunosuppression withdrawal in liver transplant recipients: role of ursodeoxycholic acid. Transplantation 83, 1571–1576 (2007).
Zhao, X. et al. Intragraft Vδ1 γδ T cells with a unique T-cell receptor are closely associated with pediatric semiallogeneic liver transplant tolerance. Transplantation 95, 192–202 (2013).
Li, Y. et al. The presence of Foxp3 expressing T cells within grafts of tolerant human liver transplant recipients. Transplantation 86, 1837–1843 (2008).
Li, L. et al. A common peripheral blood gene set for diagnosis of operational tolerance in pediatric and adult liver transplantation. Am. J. Transpl. 12, 1218–1228 (2012).
Feng, S. et al. The primary outcome of iWITH: a multi-center clinical trial of complete immunosuppression withdrawal in stable pediatric liver transplant recipients. Am. J. Transplant. 16, 269 (2016). [abstract 354].
Donckier, V. et al. Donor stem cell infusion after non-myeloablative conditioning for tolerance induction to HLA mismatched adult living-donor liver graft. Transpl. Immunol. 13, 139–146 (2004).
Eason, J. D., Cohen, A. J., Nair, S., Alcantera, T. & Loss, G. E. Tolerance: is it worth the risk? Transplantation 79, 1157–1159 (2005).
Tryphonopoulos, P. et al. The role of donor bone marrow infusions in withdrawal of immunosuppression in adult liver allotransplantation. Am. J. Transpl. 5, 608–613 (2005).
Tryphonopoulos, P. et al. Long-term follow-up of 23 operational tolerant liver transplant recipients. Transplantation 90, 1556–1561 (2010).
Benitez, C. E. et al. ATG-Fresenius treatment and low-dose tacrolimus: results of a randomized controlled trial in liver transplantation. Am. J. Transpl. 10, 2296–2304 (2010).
Sanchez-Fueyo, A. et al. Applicability, safety, and biological activity of regulatory T cell therapy in liver transplantation. Am. J. Transplant. https://doi.org/10.1111/ajt.15700 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02166177 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02474199 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02549586 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02949492 (2019).
D’Antiga, L. (Ed.) Pediatric Hepatology and Liver Transplantation (Springer, 2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00597935 (2018).
Acknowledgements
The authors’ work is supported by National Institutes of Health grants R01 AI 118777, U19 AI 131453 and U01 AI 136779 (A.W.T), the Medical Research Council Centre for Transplantation and the National Institute for Health Research Biomedical Research Centre based at Guy’s and St Thomas’ National Health Service Foundation Trust and King’s College London (A.S.-F.) and the Swiss National Science Foundation grant P2LAP3_181318 (J.V.). The authors thank M. Freeman for skilful administrative support.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks P. Knolle and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Thomson, A.W., Vionnet, J. & Sanchez-Fueyo, A. Understanding, predicting and achieving liver transplant tolerance: from bench to bedside. Nat Rev Gastroenterol Hepatol 17, 719–739 (2020). https://doi.org/10.1038/s41575-020-0334-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-020-0334-4
This article is cited by
-
Immune disguise: the mechanisms of Neu5Gc inducing autoimmune and transplant rejection
Genes & Immunity (2022)
-
Advances in single-cell sequencing: insights from organ transplantation
Military Medical Research (2021)
-
The immunological and metabolic landscape in primary and metastatic liver cancer
Nature Reviews Cancer (2021)