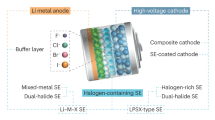
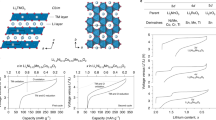
Abstract
Halogen-powered static conversion batteries (HSCBs) thrive in energy storage applications. They fall into the category of secondary non-flow batteries and operate by reversibly changing the chemical valence of halogens in the electrodes or/and electrolytes to transfer electrons, distinguishing them from the classic rocking-chair batteries. The active halide chemicals developed for these purposes include organic halides, halide salts, halogenated inorganics, organic–inorganic halides and the most widely studied elemental halogens. Aside from this, various redox mechanisms have been discovered based on multi-electron transfer and effective reaction pathways, contributing to improved electrochemical performances and stabilities of HSCBs. In this Review, we discuss the status of HSCBs and their electrochemical mechanism–performance correlations. We first provide a detailed exposition of the fundamental redox mechanisms, thermodynamics, conversion and catalysis chemistry, and mass or electron transfer modes involved in HSCBs. We conclude with a perspective on the challenges faced by the community and opportunities towards practical applications of high-energy halogen cathodes in energy-storage devices.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Armand, M. & Tarascon, J. M. Building better batteries. Nature 451, 652–657 (2008).
Simon, P. & Gogotsi, Y. Perspectives for electrochemical capacitors and related devices. Nat. Mater. 19, 1151–1163 (2020).
Xu, K. Li-ion battery electrolytes. Nat. Energy 6, 763 (2021).
Sun, Y. M., Liu, N. A. & Cui, Y. Promises and challenges of nanomaterials for lithium-based rechargeable batteries. Nat. Energy 1, 16071 (2016).
Liu, K., Liu, Y., Lin, D., Pei, A. & Cui, Y. Materials for lithium-ion battery safety. Sci. Adv. 4, eaas9820 (2018).
Nitta, N., Wu, F. X., Lee, J. T. & Yushin, G. Li-ion battery materials: present and future. Mater. Today 18, 252–264 (2015).
Jiao, S. H. et al. Stable cycling of high-voltage lithium metal batteries in ether electrolytes. Nat. Energy 3, 739–746 (2018).
Duffner, F. et al. Post-lithium-ion battery cell production and its compatibility with lithium-ion cell production infrastructure. Nat. Energy 6, 123–134 (2021).
Cui, J., Zheng, H. & He, K. J. A. M. In situ TEM study on conversion‐type electrodes for rechargeable ion batteries. Adv. Mater. 33, 2000699 (2021).
Dai, C. et al. Enabling fast-charging selenium-based aqueous batteries via conversion reaction with copper ions. Nat. Commun. 13, 1863 (2022).
Wei, S. et al. A stable room-temperature sodium–sulfur battery. Nat. Commun. 7, 11722 (2016).
Zhou, J. W., Liu, Y., Zhang, S. L., Zhou, T. F. & Guo, Z. P. Metal chalcogenides for potassium storage. InfoMat 2, 437–465 (2020).
Geels, F. W., Sovacool, B. K., Schwanen, T. & Sorrell, S. The socio-technical dynamics of low-carbon transitions. Joule 1, 463–479 (2017).
Liang, G., Mo, F., Ji, X. & Zhi, C. Non-metallic charge carriers for aqueous batteries. Nat. Rev. Mater. 6, 109–123 (2021). A comprehensive review of the regulation of electrolytes and anion chemistry.
Zhao, X., Zhao-Karger, Z., Fichtner, M. & Shen, X. Halide-based materials and chemistry for rechargeable batteries. Angew. Chem. Int. Ed. 59, 5902–5949 (2020).
She, L. et al. Rechargeable aqueous zinc–halogen batteries: fundamental mechanisms, research issues, and future perspectives. Adv. Sci. 11, 2305061 (2024).
Birk, J. Development of the Zinc–Chlorine Battery for Utility Applications. Interim report, January 1, 1977–March 31, 1978. [100-MWh plant with 45-kWh modules] (Energy Development Associates, 1979).
Xue, Z. Y., Gao, Z. Y. & Zhao, X. Y. Halogen storage electrode materials for rechargeable batteries. Energy Environ. Mater. 5, 1155–1179 (2022).
Gutmann, F., Hermann, A. & Rembaum, A. Solid‐state electrochemical cells based on charge transfer complexes. J. Electrochem. Soc. 114, 323 (1967).
Zhao, Y., Wang, L. & Byon, H. R. High-performance rechargeable lithium-iodine batteries using triiodide/iodide redox couples in an aqueous cathode. Nat. Commun. 4, 1896 (2013).
Yang, C. et al. Aqueous Li-ion battery enabled by halogen conversion–intercalation chemistry in graphite. Nature 569, 245–250 (2019). This is the first study demonstrating the intercalation and co-conversion chemistry of Br–Cl in interhalogen mode with ‘water-in-salt’ electrolytes.
Zhu, G. et al. Rechargeable Na/Cl2 and Li/Cl2 batteries. Nature 596, 525–530 (2021). This is the first study demonstrating the rechargeable Li-SOCl2 battery operating via redox between mainly Cl2 and Cl−.
Zou, Y. et al. A four-electron Zn-I2 aqueous battery enabled by reversible I−/I2/I+ conversion. Nat. Commun. 12, 170 (2021). This is the first study demonstrating the two-electron transfer chemistry of zinc-iodine batteries in aqueous/organic hybrid electrolytes.
Li, X. et al. Two-electron redox chemistry enabled high-performance iodide-ion conversion battery. Angew. Chem. Int. Ed. 61, e202113576 (2022).
Ma, W. et al. A twelve-electron conversion iodine cathode enabled by interhalogen chemistry in aqueous solution. Nat. Commun. 14, 5508 (2023). This paper realizes the multi-electron transfer mode in hetero-halogen electrolytes with a I−/IO3− redox couple.
He, B. et al. Halogen chemistry of solid electrolytes in all-solid-state batteries. Nat. Rev. Chem. 7, 826–842 (2023). This paper comprehensively summarizes halogen-based solid-state electrolytes.
Schwerdtfeger, P., Smits, O. R. & Pyykko, P. The periodic table and the physics that drives it. Nat. Rev. Chem. 4, 359–380 (2020).
Politzer, P., Lane, P., Concha, M. C., Ma, Y. & Murray, J. S. An overview of halogen bonding. J. Mol. Model. 13, 305–311 (2007).
Kirk, K. L. in Biochemistry of the Elemental Halogens Inorganic Halides (ed. Kirk, K. L.) 1–17 (Springer, 1991).
Tang, X., Fan, T., Wang, C. & Zhang, H. Halogen functionalization in the 2D material flatland: strategies, properties, and applications. Small 17, e2005640 (2021).
Solel, E., Ruth, M. & Schreiner, P. R. London dispersion helps refine steric A-values: the halogens. J. Org. Chem. 86, 7701–7713 (2021).
Pennington, W. T., Hanks, T. W. & Arman, H. D. in Halogen Bonding. Structure and Bonding (eds Metrangolo, P. & Resnati, G.) 65–104 (Springer, 2007).
Zhou, F. et al. A new type of halogen bond involving multivalent astatine: an ab initio study. Phys. Chem. Chem. Phys. 21, 15310–15318 (2019).
Ji, X. A perspective of ZnCl2 electrolytes: the physical and electrochemical properties. eScience 1, 99–107 (2021).
Guo, Q. et al. Reversible insertion of I–Cl interhalogen in a graphite cathode for aqueous dual-ion batteries. ACS Energy Lett. 6, 459–467 (2021).
Chun, S. E. et al. Design of aqueous redox-enhanced electrochemical capacitors with high specific energies and slow self-discharge. Nat. Commun. 6, 7818 (2015).
Zhao, Y. et al. A chemistry and material perspective on lithium redox flow batteries towards high-density electrical energy storage. Chem. Soc. Rev. 44, 7968–7996 (2015).
Boettcher, S. W. et al. Potentially confusing: potentials in electrochemistry. ACS Energy Lett. 6, 261–266 (2021).
Zhang, J. et al. An all-aqueous redox flow battery with unprecedented energy density. Energy Environ. Sci. 11, 2010–2015 (2018).
Liu, Z. et al. Voltage issue of aqueous rechargeable metal-ion batteries. Chem. Soc. Rev. 49, 180–232 (2020).
Fan, X. & Wang, C. High-voltage liquid electrolytes for Li batteries: progress and perspectives. Chem. Soc. Rev. 50, 10486–10566 (2021).
Wang, Z., Meng, X., Chen, K. & Mitra, S. High capacity aqueous periodate batteries featuring a nine-electron transfer process. Energy Storage Mater. 19, 206–211 (2019).
Zhang, K. & Jin, Z. Halogen-enabled rechargeable batteries: current advances and future perspectives. Energy Storage Mater. 45, 332–369 (2022).
Lu, K. et al. A rechargeable iodine-carbon battery that exploits ion intercalation and iodine redox chemistry. Nat. Commun. 8, 527 (2017).
Qian, M. et al. Realizing few-layer iodinene for high-rate sodium-ion batteries. Adv. Mater. 32, e2004835 (2020).
Sui, Y. et al. Reversible Cl2/Cl− redox for low-temperature aqueous batteries. ACS Energy Lett. 8, 988–994 (2023). This paper reports the rechargeable aqueous Cl battery at an ultra-low temperature of −78 °C.
Biswas, S. et al. Minimal architecture zinc–bromine battery for low cost electrochemical energy storage. Energy Environ. Sci. 10, 114–120 (2017).
Li, X. et al. Enhanced redox kinetics and duration of aqueous I2/I− conversion chemistry by MXene confinement. Adv. Mater. 33, e2006897 (2021).
Wu, W. L. et al. A polybromide confiner with selective bromide conduction for high performance aqueous zinc-bromine batteries. Energy Storage Mater. 49, 11–18 (2022).
Li, X. et al. Confining aqueous Zn–Br halide redox chemistry by Ti3C2TX MXene. ACS Nano 15, 1718–1726 (2021).
Brown, R. F. Electronegativity, non-bonded interactions and polarizability in the hydrogen halides and the interhalogen compounds. J. Am. Chem. Soc. 83, 36–42 (2002).
Lv, S. et al. A high-performance quasi-solid-state aqueous zinc-dual halogen battery. ACS Nano 16, 20389–20399 (2022).
Chen, S. et al. Solid interhalogen compounds with effective Br0 fixing for stable high‐energy zinc batteries. Angew. Chem. Int. Ed. 62, e202301467 (2023).
Gao, T. et al. Povidone-iodine-based polymeric nanoparticles for antibacterial applications. ACS Appl. Mater. Interfaces 9, 25738–25746 (2017).
Gao, L. et al. A high-performance aqueous zinc-bromine static battery. iScience 23, 101348 (2020).
Li, X. et al. Three-electron transfer-based high-capacity organic lithium-iodine (chlorine) batteries. Angew. Chem. Int. Ed. 62, e202310168 (2023).
Sun, Y., Zou, Q. & Lu, Y. C. Fast and reversible four‐electron storage enabled by ethyl viologen for rechargeable magnesium batteries. Adv. Energy Mater. 9, 1903002 (2019).
Li, X. L. et al. Bis-ammonium salts with strong chemisorption to halide ions for fast and durable aqueous redox Zn ion batteries. Nano Energy 98, 107278 (2022). This is the first study demonstrating the halogen-powered static conversion chemistry of perovskite materials.
Méndez-Díaz, J. D. et al. Sunlight-driven photochemical halogenation of dissolved organic matter in seawater: a natural abiotic source of organobromine and organoiodine. Environ. Sci. Technol. 48, 7418–7427 (2014).
Kim, S., Kim, S. K., Sun, P., Oh, N. & Braun, P. V. Reduced graphene oxide/LiI composite lithium ion battery cathodes. Nano Lett. 17, 6893–6899 (2017).
Liu, H. et al. A zinc–dual-halogen battery with a molten hydrate electrolyte. Adv. Mater. 32, e2004553 (2020).
Xia, M. T. et al. A rechargeable K/Br battery. Adv. Funct. Mater. 32, 2205879 (2022).
Hong, J. J. et al. A dual plating battery with the iodine/[ZnIx(OH2)4−x]2−x cathode. Angew. Chem. Int. Ed. 58, 15910–15915 (2019).
Li, X. et al. Perovskite cathodes for aqueous and organic iodine batteries operating under one and two electrons redox modes. Adv. Mater. 36, 2304557 (2023).
Wang, S. et al. Conversion‐type organic‐inorganic tin‐based perovskite cathodes for durable aqueous zinc‐iodine batteries. Adv. Energy Mater. 13, 2300922 (2023).
Park, M. et al. Edge-halogenated graphene nanoplatelets with F, Cl, or Br as electrocatalysts for all-vanadium redox flow batteries. Nano Energy 26, 233–240 (2016).
Li, M. et al. Element replacement approach by reaction with Lewis acidic molten salts to synthesize nanolaminated MAX phases and MXenes. J. Am. Chem. Soc. 141, 4730–4737 (2019).
Li, Y. et al. A general Lewis acidic etching route for preparing MXenes with enhanced electrochemical performance in non-aqueous electrolyte. Nat. Mater. 19, 894–899 (2020).
Li, X. et al. Activating the I0/I+ redox couple in an aqueous I2–Zn battery to achieve a high voltage plateau. Energy Environ. Sci. 14, 407–413 (2021).
Kamysbayev, V. et al. Covalent surface modifications and superconductivity of two-dimensional metal carbide MXenes. Science 369, 979–983 (2020).
Li, M. et al. Halogenated Ti3C2 MXenes with electrochemically active terminals for high-performance zinc ion batteries. ACS Nano 15, 1077–1085 (2021). This is the first study demonstrating the conversion chemistry of halogenated MXenes.
Yao, N., Chen, X., Fu, Z. H. & Zhang, Q. Applying classical, ab initio, and machine-learning molecular dynamics simulations to the liquid electrolyte for rechargeable batteries. Chem. Rev. 122, 10970–11021 (2022).
Tang, H. et al. Unleashing energy storage ability of aqueous battery electrolytes. Mater. Futures 1, 022001 (2022).
Li, X. et al. Intrinsic voltage plateau of a Nb2CTx MXene cathode in an aqueous electrolyte induced by high-voltage scanning. Joule 5, 2993–3005 (2021).
Sun, Q. J. et al. Interfacial and interphasial chemistry of electrolyte components to invoke high-performance antimony anodes and non-flammable lithium-ion batteries. Adv. Funct. Mater. 33, 2210292 (2023).
Yang, S. et al. Rechargeable iodine batteries: fundamentals, advances, and perspectives. ACS Nano 16, 13554–13572 (2022).
Liu, H. et al. High sulfur loading and shuttle inhibition of advanced sulfur cathode enabled by graphene network skin and N, P, F-doped mesoporous carbon interfaces for ultra-stable lithium sulfur battery. Nano Res. Energy 2, e9120049 (2023).
Prehal, C. et al. Persistent and reversible solid iodine electrodeposition in nanoporous carbons. Nat. Commun. 11, 4838 (2020).
Liu, M. et al. Physicochemical confinement effect enables high-performing zinc–iodine batteries. J. Am. Chem. Soc. 144, 21683–21691 (2022).
Zhao, Q., Lu, Y., Zhu, Z., Tao, Z. & Chen, J. Rechargeable lithium-iodine batteries with iodine/nanoporous carbon cathode. Nano Lett. 15, 5982–5987 (2015).
Li, X. et al. MXene chemistry, electrochemistry and energy storage applications. Nat. Rev. Chem. 6, 389–404 (2022).
Lee, J. H. et al. High-energy efficiency membraneless flowless Zn–Br battery: utilizing the electrochemical–chemical growth of polybromides. Adv. Mater. 31, e1904524 (2019).
Guo, C. et al. Hydrogen-bonded organic framework for high-performance lithium/sodium-iodine organic batteries. Angew. Chem. Int. Ed. 61, e202213276 (2022).
Wang, F. et al. Fully conjugated phthalocyanine copper metal–organic frameworks for sodium–iodine batteries with long‐time‐cycling durability. Adv. Mater. 32, 1905361 (2020).
Ma, L. et al. Electrocatalytic iodine reduction reaction enabled by aqueous zinc-iodine battery with improved power and energy densities. Angew. Chem. Int. Ed. 60, 3791–3798 (2021). This paper reports how the catalytic sites in hosts catalyse the conversion chemistry of iodine batteries.
Yang, Y., Liang, S. & Zhou, J. Progress and prospect of the zinc–iodine battery. Curr. Opin. Electrochem. 30, 100761 (2021).
Gong, D. et al. An iodine quantum dots based rechargeable sodium–iodine battery. Adv. Energy Mater. 7, 1601885 (2017).
Zhang, Z., Zhu, Y., Yu, M., Jiao, Y. & Huang, Y. Development of long lifespan high-energy aqueous organic||iodine rechargeable batteries. Nat. Commun. 13, 6489 (2022).
Li, K. et al. A 3D and stable lithium anode for high-performance lithium–iodine batteries. Adv. Mater. 31, e1902399 (2019).
Tian, H. J., Zhang, S. L., Meng, Z., He, W. & Han, W. Q. Rechargeable aluminum/iodine battery redox chemistry in ionic liquid electrolyte. ACS Energy Lett. 2, 1170–1176 (2017).
Shen, Z. et al. Electrocrystallization regulation enabled stacked hexagonal platelet growth toward highly reversible zinc anodes. Angew. Chem. Int. Ed. 62, e202218452 (2023).
Zhang, Y. et al. Iodine promoted ultralow Zn nucleation overpotential and Zn-rich cathode for low-cost, fast-production and high-energy density anode-free Zn-iodine batteries. Nano Micro Lett. 14, 208 (2022).
Wang, Y. et al. Electrolyte design for rechargeable anion shuttle batteries. eScience 2, 573–590 (2022).
Jin, C. B. et al. Rejuvenating dead lithium supply in lithium metal anodes by iodine redox. Nat. Energy 6, 378–387 (2021). This paper reports a lithium restoration method based on a series of iodine redox reactions mainly involving I3−/I− in lithium metal anodes.
Liu, M. Z. et al. Deciphering the paradox between the Co-intercalation of sodium-solvent into graphite and its irreversible capacity. Energy Storage Mater. 26, 32–39 (2020).
Goktas, M. et al. Graphite as cointercalation electrode for sodium-ion batteries: electrode dynamics and the missing solid electrolyte interphase (SEI). Adv. Energy Mater. 8, 1702724 (2018).
Zhang, Q. et al. Halogenated Zn2+ solvation structure for reversible Zn metal batteries. J. Am. Chem. Soc. 144, 18435–18443 (2022).
Cao, J. et al. Strategies of regulating Zn2+ solvation structures for dendrite-free and side reaction-suppressed zinc-ion batteries. Energy Environ. Sci. 15, 499–528 (2022).
Li, C. et al. Tuning the solvation structure in aqueous zinc batteries to maximize Zn-ion intercalation and optimize dendrite-free zinc plating. ACS Energy Lett. 7, 533–540 (2022).
Wang, X. et al. Electrode material–ionic liquid coupling for electrochemical energy storage. Nat. Rev. Mater. 5, 787–808 (2020).
Yang, X. et al. Electrolyte design principles for developing quasi-solid-state rechargeable halide-ion batteries. Nat. Commun. 14, 925 (2023).
Kim, K. I. et al. Reversible insertion of Mg-Cl superhalides in graphite as a cathode for aqueous dual-ion batteries. Angew. Chem. Int. Ed. 59, 19924–19928 (2020).
Huang, Z. et al. Anion chemistry in energy storage devices. Nat. Rev. Chem. 7, 616–631 (2023).
Hou, S. et al. High-energy and low-cost membrane-free chlorine flow battery. Nat. Commun. 13, 1281 (2022).
Yang, Y., Liang, S., Lu, B., Zhou, J. J. E. & Science, E. Eutectic electrolyte based on N-methylacetamide for highly reversible zinc–iodine battery. Energy Environ. Sci. 15, 1192–1200 (2022).
Zhu, G. et al. High-capacity rechargeable Li/Cl2 batteries with graphite positive electrodes. J. Am. Chem. Soc. 144, 22505–22513 (2022).
Dai, C. et al. Fast constructing polarity-switchable zinc-bromine microbatteries with high areal energy density. Sci. Adv. 8, eabo6688 (2022).
Sonigara, K. K., Zhao, J., Machhi, H. K., Cui, G. & Soni, S. S. Self‐assembled solid‐state gel catholyte combating iodide diffusion and self‐discharge for a stable flexible aqueous Zn–I2 battery. Adv. Energy Mater. 10, 2001997 (2020).
Suo, L. et al. “Water-in-salt” electrolyte enables high-voltage aqueous lithium-ion chemistries. Science 350, 938–943 (2015).
Xie, J., Liang, Z. & Lu, Y. C. Molecular crowding electrolytes for high-voltage aqueous batteries. Nat. Mater. 19, 1006–1011 (2020).
Chen, F., Wang, X., Armand, M. & Forsyth, M. Cationic polymer-in-salt electrolytes for fast metal ion conduction and solid-state battery applications. Nat. Mater. 21, 1175–1182 (2022).
Wang, F., Sun, Y. & Cheng, J. Switching of redox levels leads to high reductive stability in water-in-salt electrolytes. J. Am. Chem. Soc. 145, 4056–4064 (2023).
Wu, Z. et al. Deciphering and modulating energetics of solvation structure enables aggressive high-voltage chemistry of Li metal batteries. Chem 9, 650–664 (2023).
Wang, D. et al. A thermodynamic cycle-based electrochemical windows database of 308 electrolyte solvents for rechargeable batteries. Adv. Funct. Mater. 33, 2212342 (2023).
Li, M. et al. Design strategies for nonaqueous multivalent-ion and monovalent-ion battery anodes. Nat. Rev. Mater. 5, 276–294 (2020).
Sun, W. et al. A rechargeable zinc-air battery based on zinc peroxide chemistry. Science 371, 46–51 (2021).
Zhang, S. J. et al. Polyiodide confinement by starch enables shuttle-free Zn–iodine batteries. Adv. Mater. 34, e2201716 (2022).
Li, P., Li, C., Guo, X., Li, X. & Zhi, C. Metal-iodine and metal-bromine batteries: a review. Bull. Chem. Soc. Jpn 94, 2036–2042 (2021).
Yoo, S. J. et al. Fundamentally addressing bromine storage through reversible solid-state confinement in porous carbon electrodes: design of a high-performance dual-redox electrochemical capacitor. J. Am. Chem. Soc. 139, 9985–9993 (2017).
Bai, C. et al. A sustainable aqueous Zn-I2 battery. Nano Res. 11, 3548–3554 (2018).
Xu, J. et al. Lithium halide cathodes for Li metal batteries. Joule 7, 83–94 (2023). This paper reports the remarkable universality of lithium halide cathodes for Li metal batteries.
Yang, H. et al. A metal–organic framework as a multifunctional ionic sieve membrane for long-life aqueous zinc–iodide batteries. Adv. Mater. 32, e2004240 (2020).
Wu, X. & Ji, X. Aqueous batteries get energetic. Nat. Chem. 11, 680–681 (2019).
Wang, A. & Gyenge, E. L. Borohydride electro-oxidation in a molten alkali hydroxide eutectic mixture and a novel borohydride–periodate battery. J. Power Sources 282, 169–173 (2015).
Zhang, F., Wang, Q. & Tang, Y. B. Extended iodine chemistry: toward high-energy-density aqueous zinc-ion batteries. Matter 4, 2637–2639 (2021).
Liang, G. et al. Development of rechargeable high-energy hybrid zinc-iodine aqueous batteries exploiting reversible chlorine-based redox reaction. Nat. Commun. 14, 1856 (2023).
Cai, S. et al. Water–salt oligomers enable supersoluble electrolytes for high-performance aqueous batteries. Adv. Mater. 33, e2007470 (2021).
Zhou, G., Chen, H. & Cui, Y. Formulating energy density for designing practical lithium–sulfur batteries. Nat. Energy 7, 312–319 (2022).
Lu, Y. & Chen, J. Prospects of organic electrode materials for practical lithium batteries. Nat. Rev. Chem. 4, 127–142 (2020).
Liu, B., Zhang, J. G. & Xu, W. Advancing lithium metal batteries. Joule 2, 833–845 (2018).
Xu, J., Ma, J., Fan, Q., Guo, S. & Dou, S. Recent progress in the design of advanced cathode materials and battery models for high-performance lithium-X (X = O2, S, Se, Te, I2, Br2) batteries. Adv. Mater. 29, 1606454 (2017).
Zhao, Y. et al. Few-layer bismuth selenide cathode for low-temperature quasi-solid-state aqueous zinc metal batteries. Nat. Commun. 13, 752 (2022).
Wang, F. et al. Highly reversible zinc metal anode for aqueous batteries. Nat. Mater. 17, 543–549 (2018).
Li, X. et al. Phase transition induced unusual electrochemical performance of V2CTX MXene for aqueous zinc hybrid-ion battery. ACS Nano 14, 541–551 (2020).
Wan, F. & Niu, Z. Design strategies for vanadium-based aqueous zinc-ion batteries. Angew. Chem. Int. Ed. 58, 16358–16367 (2019).
Xie, J. & Lu, Y. C. A retrospective on lithium-ion batteries. Nat. Commun. 11, 2499 (2020).
Suo, L. et al. Fluorine-donating electrolytes enable highly reversible 5-V-class Li metal batteries. Proc. Natl Acad. Sci. USA 115, 1156–1161 (2018).
Tian, R. et al. Quantifying the factors limiting rate performance in battery electrodes. Nat. Commun. 10, 1933 (2019).
Wang, J. et al. Superconcentrated electrolytes for a high-voltage lithium-ion battery. Nat. Commun. 7, 12032 (2016).
Tang, X. et al. High-performance quasi-solid-state MXene-based Li–I batteries. ACS Cent. Sci. 5, 365–373 (2019).
Liang, G. et al. Regulating inorganic and organic components to build amorphous‐ZnFx enriched solid‐electrolyte interphase for highly reversible Zn metal chemistry. Adv. Mater. 35, 2210051 (2023).
Xu, J. et al. Electrolyte design for Li-ion batteries under extreme operating conditions. Nature 614, 694–700 (2023).
Jiang, L., Dong, D. & Lu, Y.-C. Design strategies for low temperature aqueous electrolytes. Nano Res. Energy 1, e9120003 (2022).
Wan, H., Xu, J. & Wang, C. Designing electrolytes and interphases for high-energy lithium batteries. Nat. Rev. Chem. 8, 30–44 (2023).
Zhao, C., Lu, Y., Chen, L. & Hu, Y. S. Flexible Na batteries. InfoMat 2, 126–138 (2019).
Jin, X. et al. A flexible aqueous zinc–iodine microbattery with unprecedented energy density. Adv. Mater. 34, e2109450 (2022). This paper reports a flexible aqueous zinc–iodine microbattery and its application in mechanical deformation scenarios.
Lu, J., Wu, T. & Amine, K. J. N. E. State-of-the-art characterization techniques for advanced lithium-ion batteries. Nat. Energy 2, 1–13 (2017).
Zhou, S. et al. Visualizing interfacial collective reaction behaviour of Li–S batteries. Nature 621, 75–81 (2023).
Pastor, E. et al. Complementary probes for the electrochemical interface. Nat. Rev. Chem. 8, 159–178 (2024).
Han, M. et al. Electrochemically modulated interaction of MXenes with microwaves. Nat. Nanotechnol. 18, 373–379 (2023).
Li, X., Li, M., Li, X., Fan, X. & Zhi, C. Low infrared emissivity and strong stealth of Ti-based MXenes. Research 2022, 9892628 (2022).
Gao, X. W. et al. Solid-state lithium battery cathodes operating at low pressures. Joule 6, 636–646 (2022).
Cheng, Z. et al. Achieving long cycle life for all-solid-state rechargeable Li-I2 battery by a confined dissolution strategy. Nat. Commun. 13, 125 (2022). This paper reports an all-solid-state rechargeable Li–I2 battery with a hybrid electrolyte.
Lu, K. et al. Rechargeable potassium-ion batteries enabled by potassium-iodine conversion chemistry. Energy Storage Mater. 16, 1–5 (2019).
Yang, S. et al. High-rate aqueous aluminum-ion batteries enabled by confined iodine conversion chemistry. Small Methods 5, e2100611 (2021).
Pan, H. et al. Controlling solid–liquid conversion reactions for a highly reversible aqueous zinc–iodine battery. ACS Energy Lett. 2, 2674–2680 (2017).
Bai, C., Jin, H., Gong, Z., Liu, X. & Yuan, Z. A high-power aqueous rechargeable Fe-I2 battery. Energy Storage Mater. 28, 247–254 (2020).
Zhang, Y. et al. A high-performance COF-based aqueous zinc-bromine battery. Chem. Eng. J. 451, 138915 (2023).
Li, P. et al. Development of an energy-dense and high-power Li-Cl2 battery using reversible interhalogen bonds. Chem 10, 352–364 (2024).
Li, P. et al. Highly thermally/electrochemically stable I−/I3− bonded organic salts with high I content for long‐life Li–I2 batteries. Adv. Energy Mater. 12, 2103648 (2022).
Meng, Z. et al. Ultra-stable binder-free rechargeable Li/I2 batteries enabled by “Betadine” chemical interaction. Chem. Commun. 54, 12337–12340 (2018).
Peterson, B. B., Andrews, E. M., Hung, F. & Flake, J. C. Carbonized metal-organic framework cathodes for secondary lithium-bromine batteries. J. Power Sources 492, 229658 (2021).
Acknowledgements
C.Z. discloses support for the research of this work from the National Key R&D Program of China [2019YFA0705104], City University of Hong Kong [9667165], and Research Grants Council [R5019-22]. X.L. discloses support for the research of this work from the State Key Laboratory of Solidification Processing in Northwestern Polytechnical University [SKLSP202312].
Author information
Authors and Affiliations
Contributions
All authors contributed to the writing and editing of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Active halide chemicals
-
Chemical substances that contain halogens and in which the halogens perform conversion-type electrochemical reactions.
- Electrochemical stability window
-
(ESW). A quantified potential range in which applicable electrolytes have the ability to resist decomposition.
- Host
-
A conductive substance that carries active halide chemicals and transfers electrons.
- Redox intermediates
-
A term denoting halogen-containing products that form during redox reactions but are not among the initial and final active chemicals.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Xu, W. & Zhi, C. Halogen-powered static conversion chemistry. Nat Rev Chem (2024). https://doi.org/10.1038/s41570-024-00597-z
Accepted:
Published:
DOI: https://doi.org/10.1038/s41570-024-00597-z