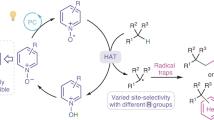
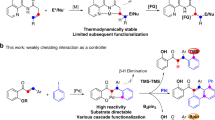
Abstract
Phthalimide-N-oxyl (PINO) is a valuable hydrogen-atom-transfer (HAT) catalyst for selective C–H functionalization. To advance and optimize PINO-catalysed HAT reactions, researchers have been focused on modifying the phthalimide core structure. Despite much effort and some notable advances, the modifications to date have centred on optimization of a single parameter of the catalyst, such as reactivity, solubility or stability. Unfortunately, the optimization with respect to one parameter is often associated with a worsening of the others. The derivation of a single catalyst structure with optimal performance across multiple parameters has therefore remained elusive. Here we present an analysis of the structure–activity relationships of PINO and its derivatives as HAT catalysts, which we hope will stimulate further development of PINO-catalysed HAT reactions and, ultimately, lead to much improved catalysts for real-world applications.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Capaldo, L., Ravelli, D. & Fagnoni, M. Direct photocatalyzed hydrogen atom transfer (HAT) for aliphatic C–H bonds elaboration. Chem. Rev. 122, 1875–1924 (2022). This comprehensive survey summarizes the synthetic applications of photocatalysed HAT mediated by a variety of reagents.
Cao, H., Tang, X., Tang, H., Yuan, Y. & Wu, J. Photoinduced intermolecular hydrogen atom transfer reactions in organic synthesis. Chem. Catal. 1, 523–598 (2021).
Bosque, I., Magallanes, G., Rigoulet, M., Kärkäs, M. D. & Stephenson, C. R. J. Redox catalysis facilitates lignin depolymerization. ACS Cent. Sci. 3, 621–628 (2017).
Horn, E. J. et al. Scalable and sustainable electrochemical allylic C–H oxidation. Nature 533, 77–81 (2016).
Recupero, F. & Punta, C. Free radical functionalization of organic compounds catalyzed by N-hydroxyphthalimide. Chem. Rev. 107, 3800–3842 (2007). This Review provides a detailed summary of NHPI/PINO-mediated C–H functionalization.
Amorati, R. et al. Hydroxylamines as oxidation catalysts: thermochemical and kinetic studies. J. Org. Chem. 68, 1747–1754 (2003). This high-impact physical organic study describes both thermodynamic and kinetic aspects of different types of nitroxide.
Melone, L. & Punta, C. Metal-free aerobic oxidations mediated by N-hydroxyphthalimide. A concise review. Beilstein J. Org. Chem. 9, 1296–1310 (2013).
Bera, A., Bera, S. & Banerjee, D. Recent advances in the synthesis of N-heteroarenes via catalytic dehydrogenation of N-heterocycles. Chem. Commun. 57, 13042–13058 (2021).
Andrade, M. A. & Martins, L. M. D. R. S. Organocatalysis meets hydrocarbon oxyfunctionalization: the role of N-hydroxyimides. Eur. J. Org. Chem. 2021, 4715–4727 (2021).
Lang, X. & Zhao, J. Integrating TEMPO and its analogues with visible-light photocatalysis. Chem. Asian J. 13, 599–613 (2018).
Melone, L. & Punta, C. N-Hydroxyphthalimide (NHPI) organocatalyzed aerobic oxidations: advantages, limits, and industrial perspectives. In Liquid Phase Aerobic Oxidation Catalysis: Industrial Applications And Academic Perspectives (eds Stahl, S. S. & Alsters, P. L.) (Wiley, 2016).
Liang, Y. & Jiao, N. Oxygenation via C–H/C–C bond activation with molecular oxygen. Acc. Chem. Res. 50, 1640–1653 (2017).
Melone, L. & Punta, C. Co-oxidation processes promoted by N-hydroxyphthalimide/aldehyde system. In New Developments In Aldehydes Research 121–138 (Nova Publishers, 2013).
Coseri, S. Phthalimide-N-oxyl (PINO) radical, a powerful catalytic agent: its generation and versatility towards various organic substrates. Catal. Rev. 51, 218–292 (2009).
Coseri, S. N-hydroxyphthalimide (NHPI)/lead tetraacetate, a peculiar system for the phthalimide-N-oxyl (PINO) radical generation. Mini-Rev. Org. Chem. 5, 222–227 (2008).
Xu, H., Tang, R., Gong, N., Liu, C. & Zhou, Y. Aerobic oxidation reactions catalyzed by N-hydroxyphthalimide and its analogues. Prog. Chem. 19, 1736–1745 (2007).
Ishii, Y. & Sakaguchi, S. Recent progress in aerobic oxidation of hydrocarbons by N-hydroxyimides. Catal. Today 117, 105–113 (2006).
Sheldon, R. A. & Arends, I. W. C. E. Catalytic oxidations mediated by metal ions and nitroxyl radicals. J. Mol. Catal. A 251, 200–214 (2006).
Liang, J., Li, J., Zhou, B. & Qin, S. Recent advance of N-hydroxyphthalimide (NHPI) in organic oxidation reaction. Chem. Res. Appl. 16, 597–600 (2004).
Tong, J., Li, Z. & Xia, C. Review on environmentally friendly catalytic oxidation system. Prog. Chem. 17, 96–110 (2005).
Sheldon, R. A. & Arends, I. W. C. E. Organocatalytic oxidations mediated by nitroxyl radicals. Adv. Synth. Catal. 346, 1051–1071 (2004).
Ishii, Y. & Sakaguchi, S. Development of catalytic carbon radical generation and its application to organic synthesis. J. Syn. Org. Chem. Jpn 61, 1056–1064 (2003).
Minisci, F., Recupero, F., Pedulli, G. F. & Lucarini, M. Transition metal salts catalysis in the aerobic oxidation of organic compounds. Thermochemical and kinetic aspects and new synthetic developments in the presence of N-hydroxy-derivative catalysts. J. Mol. Catal. A 204–205, 63–90 (2003).
Ishii, Y. Development of catalytic carbon radical generation and its application to organic synthesis. Kagaku Kogyo 53, 37–42 (2002).
Ishii, Y., Sakaguchi, S. & Iwahama, T. Innovation of hydrocarbon oxidation with molecular oxygen and related reactions. Adv. Synth. Catal. 343, 393–427 (2001).
Ishii, Y. Development of catalytic carbon radical generation and its application to organic synthesis. J. Syn. Org. Chem. Jpn 59, 2–10 (2001).
Ishii, Y., Sakaguchi, S. & Iwahama, T. Development of novel aerobic oxidation method using N-hydroxyphthalimide as catalyst. J. Syn. Org. Chem. Jpn 57, 24–34 (1999).
Chen, K. & Xie, H. Selective aerobic oxidation promoted by highly efficient multi‐nitroxy organocatalysts. Chin. J. Catal. 38, 625–635 (2017).
Wu, Z., Hu, G. & Luan, Y. Development of N-hydroxy catalysts for C−H functionalization via hydrogen atom transfer: challenges and opportunities. ACS Catal. 12, 11716–11733 (2022).
Yoshii, T. et al. N-Hydroxybenzimidazole as a structurally modifiable platform for N-oxyl radicals for direct C–H functionalization reactions. Chem. Sci. 11, 5772–5778 (2020).
Caruso, M., Petroselli, M. & Cametti, M. Design and synthesis of multipurpose derivatives for N-hydroxyimide and NHPI-based catalysis applications. ChemistrySelect 6, 12975–12980 (2021).
Zhao, Q., Chen, K., Zhang, W., Yao, J. & Li, H. Efficient metal-free oxidation of ethylbenzene with molecular oxygen utilizing the synergistic combination of NHPI analogues. J. Mol. Catal. A 402, 79–82 (2015).
Dobras, G., Kasperczyk, K., Jurczyk, S. & Orlińska, B. N-hydroxyphthalimide supported on silica coated with ionic liquids containing CoCl2 (SCILLs) as new catalytic system for solvent-free ethylbenzene oxidation. Catalysts 10, 252 (2020).
Shi, G. et al. Covalent anchoring of N-hydroxyphthalimide on silica via robust imide bonds as a reusable catalyst for the selective aerobic oxidation of ethylbenzene to acetophenone. N. J. Chem. 45, 13441–13450 (2021).
Grochowski, E., Boleslawska, T. & Jurczak, J. Reaction of diethyl azodicarboxylate with ethers in the presence of N-hydroxyimides as catalysts. Synthesis 1977, 718–720 (1977).
Melone, L. & Punta, C. Metal-free aerobic oxidations mediated by N-hydroxyphthalimide. A concise review. Beilstein J. Org. Chem. 9, 1296–1310 (2013).
Yang, G., Ma, Y. & Xu, J. Biomimetic catalytic system driven by electron transfer for selective oxygenation of hydrocarbon. J. Am. Chem. Soc. 126, 10542–10543 (2004).
Iwahama, T., Sakaguchi, S., Nishiyama, Y. & Ishii, Y. Aerobic oxidation of alcohols to carbonyl compounds catalyzed by N-hydroxyphthalimide (NHPI) combined with Co(acac)3. Tetrahedron Lett. 36, 6923–6926 (1995).
Masui, M., Ueshima, T. & Ozaki, S. N-Hydroxyphthalimide as an effective mediator for the oxidation of alcohols by electrolysis. J. Chem. Soc. Chem. Commun. https://doi.org/10.1039/C39830000479 (1983).
Yang, C., Farmar, L. A., Pratt, D. A., Maldonado, S. & Stephenson, C. R. J. Mechanisms of electrochemical generation and decomposition of phthalimide N-oxyl. J. Am. Chem. Soc. 143, 10324–10332 (2021). This mechanistic study elucidates possible PINO decomposition mechanisms through electroanalytical and computational methodologies.
Ueda, C., Noyama, M., Ohmori, H. & Masui, M. Reactivity of phthalimide-N-oxyl: a kinetic study. Chem. Pharm. Bull. 35, 1372–1377 (1987). This pioneering study investigates the decomposition of electrochemically generated PINO.
Lin, X., Lin, L., Ye, X., Tan, C. & Jiang, Z. Aerobic oxidation of benzylic sp3 C–H bonds through cooperative visible-light photoredox catalysis of N-hydroxyimide and dicyanopyrazine. Asian J. Org. Chem. 6, 422–425 (2017).
Solomon, E. I., Sundaram, U. M. & Machonkin, T. E. Multicopper oxidases and oxygenases. Chem. Rev. 96, 2563–2606 (1996).
Warren, J. J., Tronic, T. A. & Mayer, J. M. Thermochemistry of proton-coupled electron transfer reagents and its implications. Chem. Rev. 110, 6961–7001 (2010).
Nutting, J. E., Rafiee, M. & Stahl, S. S. Tetramethylpiperidine N-oxyl (TEMPO), phthalimide N-oxyl (PINO), and related N-oxyl species: electrochemical properties and their use in electrocatalytic reactions. Chem. Rev. 118, 4834–4885 (2018).
Ozaki, S. & Masui, M. Oxidation of hydroxylamine derivatives. I. Anodic oxidation of hydroxamic acids. Chem. Pharm. Bull. 25, 1179–1185 (1977).
Punta, C., Rector, C. L. & Porter, N. A. Peroxidation of polyunsaturated fatty acid methyl esters catalyzed by N-methyl benzohydroxamic acid: a new and convenient method for selective synthesis of hydroperoxides and alcohols. Chem. Res. Toxicol. 18, 349–356 (2005).
Annunziatini, C., Gerini, M. F., Lanzalunga, O. & Lucarini, M. Aerobic oxidation of benzyl alcohols catalyzed by aryl substituted N-hydroxyphthalimides. Possible involvement of a charge-transfer complex. J. Org. Chem. 69, 3431–3438 (2004).
Baucherel, X., Arends, I. W. C. E., Ellwood, S. & Sheldon, R. A. A new catalytic system for the selective aerobic oxidation of large ring cycloalkanes to ketones. Org. Process. Res. Dev. 7, 426–428 (2003).
Baucherel, X., Gonsalvi, L., Arends, I. W. C. E., Ellwood, S. & Sheldon, R. A. Aerobic oxidation of cycloalkanes, alcohols and ethylbenzene catalyzed by the novel carbon radical chain promoter NHS (N-hydroxysaccharin). Adv. Synth. Catal. 346, 286–296 (2004).
Du, H. et al. Structure–reactivity relationships of N-hydroxysaccharin analogues as organocatalysts for aerobic oxidation. Comput. Theor. Chem. 1115, 223–228 (2017).
Ishii, Y. et al. A novel catalysis of N-hydroxyphthalimide in the oxidation of organic substrates by molecular oxygen. J. Org. Chem. 60, 3934–3935 (1995).
Arnaud, R., Milet, A., Adamo, C., Einhorn, C. & Einhorn, J. Hydrogen abstraction from ethylbenzene by imide-N-oxyl radicals with and without O2: a DFT theoretical study. J. Chem. Soc. Perkin Trans. 2, 1967–1972 (2002).
Hermans, I., Jacobs, P. & Peeters, J. Autoxidation catalysis with N-hydroxyimides: more-reactive radicals or just more radicals? Phys. Chem. Chem. Phys. 9, 686–690 (2007).
Rafiee, M., Wang, F., Hruszkewycz, D. P. & Stahl, S. S. N-hydroxyphthalimide-mediated electrochemical iodination of methylarenes and comparison to electron-transfer-initiated C–H functionalization. J. Am. Chem. Soc. 140, 22–25 (2008).
Xia, F. et al. Catalytic synthesis of 2,5-furandicarboxylic acid from concentrated 2,5-diformylfuran mediated by N-hydroxyimides under mild conditions. Chem. Asian J. 14, 3329–3334 (2019).
Bietti, M. et al. Evaluation of polar effects in hydrogen atom transfer reactions from activated phenols. J. Org. Chem. 84, 1778–1786 (2019).
Kadoh, Y., Oisaki, K. & Kanai, M. Enhanced structural variety of nonplanar N-oxyl radical catalysts and their application to the aerobic oxidation of benzylic C–H bonds. Chem. Pharm. Bull. 64, 737–753 (2016).
Ozawa, J., Tashiro, M., Ni, J., Oisaki, K. & Kanai, M. Chemo- and regioselective oxygenation of C(sp3)–H bonds in aliphatic alcohols using a covalently bound directing activator and atmospheric oxygen. Chem. Sci. 7, 1904–1909 (2016).
Zhang, Q. et al. Efficient metal-free aerobic oxidation of aromatic hydrocarbons utilizing aryl-tetrahalogenated N-hydroxyphthalimides and 1,4-diamino-2,3-dichloroanthraquinone. J. Chem. Technol. Biotechnol. 83, 1364–1369 (2008). Landmark article demonstrating a meaningful improvement of PINO’s catalytic performance through electron-withdrawing polarization.
Mazzonna, M., Bietti, M., DiLabio, G. A., Lanzalunga, O. & Salamone, M. Importance of π-stacking interactions in the hydrogen atom transfer reactions from activated phenols to short-lived N-oxyl radicals. J. Org. Chem. 79, 5209–5218 (2014).
DiLabio, G. A. et al. Hydrogen atom transfer (HAT) processes promoted by the quinolinimide-N-oxyl radical. A kinetic and theoretical study. J. Org. Chem. 82, 6133–6141 (2017).
Zhang, Q. et al. A complexation promoted organic N-hydroxy catalytic system for selective oxidation of toluene. Adv. Synth. Catal. 353, 226–230 (2011).
Gorgy, K. et al. Electrocatalytic oxidation of alcohols using substituted N-hydroxyphthalimides as catalysts. Electrochim. Acta 44, 385–393 (1998).
Kato, T. & Maruoka, K. Design of bowl-shaped N-hydroxyimide derivatives as new organoradical catalysts for site-selective C(sp3)–H bond functionalization reactions. Angew. Chem. Int. Edn 59, 14261–14264 (2020).
Kato, T. & Maruoka, K. Selective functionalization of benzylic C–H bonds of two different benzylic ethers by bowl-shaped N-hydroxyimide derivatives as efficient organoradical catalysts. Chem. Commun. 58, 1021–1024 (2022).
Einhorn, C., Einhorn, J., Marcadal-Abbadi, C. & Pierre, J. Synthesis of axially chiral N-hydroxyimides, potential new catalysts for asymmetric oxidations. J. Org. Chem. 64, 4542–4546 (1999).
Nechab, M., Kumar, D. N., Philouze, C., Einhorn, C. & Einhorn, J. Variable C2-symmetric analogues of N-hydroxyphthalimide as enantioselective catalysts for aerobic oxidation: kinetic resolution of oxazolidines. Angew. Chem. 119, 3140–3143 (2007).
Shen, J. & Tan, C. Anthrone-derived NHPI analogues as catalysts in reactions using oxygen as an oxidant. Org. Biomol. Chem. 6, 4096–4098 (2008).
Maillard, B., Ingold, K. U. & Scaiano, J. C. Rate constants for the reactions of free radicals with oxygen in solution. J. Am. Chem. Soc. 105, 5095–5099 (1983).
Sawatari, N., Yokota, T., Sakaguchi, S. & Ishii, Y. Alkane oxidation with air catalyzed by lipophilic N-hydroxyphthalimides without any solvent. J. Org. Chem. 66, 7889–7891 (2001).
Guha, S. K. et al. Aerobic oxidation of cyclohexane using N-hydroxyphthalimide bearing fluoroalkyl chains. Adv. Synth. Catal. 350, 1323–1330 (2008).
Petroselli, M., Melone, L., Cametti, M. & Punta, C. Lipophilic N-hydroxyphthalimide catalysts for the aerobic oxidation of cumene: towards solvent-free conditions and back. Chem. Eur. J. 23, 10616–10625 (2017).
Petroselli, M., Franchi, P., Lucarini, M., Punta, C. & Melone, L. Aerobic oxidation of alkylaromatics using a lipophilic N-hydroxyphthalimide: overcoming the industrial limit of catalyst solubility. ChemSusChem 7, 2695–2703 (2014).
Koshino, N., Saha, B. & Espenson, J. H. Kinetic study of the phthalimide N-oxyl radical in acetic acid. Hydrogen abstraction from substituted toluenes, benzaldehydes, and benzyl alcohols. J. Org. Chem. 68, 9364–9370 (2003).
Baciocchi, E., Gerini, M. F. & Lanzalunga, O. Reactivity of phthalimide N-oxyl radical (PINO) toward the phenolic O–H bond. A kinetic study. J. Org. Chem. 69, 8963–8966 (2004).
Kishioka, S. Electrode reaction of N-hydroxyphthalimide in sulfuric acid–acetonitrile mixed solution as a catalytic mediator for alcohol oxidation. J. Electroanal. Chem. 911, 116166 (2022).
Tian, Y. et al. Unlocking high-potential non-persistent radical chemistry for semi-aqueous redox batteries. Chem. Commun. 55, 2154–2157 (2019).
Kushch, O. et al. Kinetics of N-oxyl radicals’ decay. J. Org. Chem. 85, 7112–7124 (2020).
Nechab, M., Einhorn, C. & Einhorn, J. New aerobic oxidation of benzylic compounds: efficient catalysis by N-hydroxy-3,4,5,6-tetraphenylphthalimide (NHTPPI)/CuCl under mild conditions and low catalyst loading. Chem. Commun. https://doi.org/10.1039/B403004D (2004). This paper demonstrates a landmark attempt to improve PINO stability through steric manipulation.
Michaux, J., Poirot, R., Einhorn, J. & Bessières, B. Co(I) catalyzed yne–ene–yne [2+2+2] cycloaddition: synthesis of highly strained pentacyclic bis-lactones. A new access to tetraaryl N-hydroxyphthalimides. Tetrahedron Lett. 55, 2849–2852 (2014).
Michaux, J., Bessières, B. & Einhorn, J. Bis-ortho-metalation/silylation of unprotected o-phthalic acids: straightforward access to new silylated N-hydroxyphthalimide (NHPI) analogs. Tetrahedron Lett. 53, 48–50 (2012).
Rafiee, M., Karimi, B. & Alizadeh, S. Mechanistic study of the electrocatalytic oxidation of alcohols by TEMPO and NHPI. ChemElectroChem 1, 455–462 (2014).
O’Neil, I. A., Cleatora, E. & Tapioca, D. A. A convenient synthesis of secondary hydroxylamines. Tetrahedron Lett. 42, 8247–8249 (2001).
Griesser, M. et al. The catalytic reaction of nitroxides with peroxyl radicals and its relevance to their cytoprotective properties. J. Am. Chem. Soc. 140, 3798–3808 (2018).
Murray, R. W., Singh, M. & Rath, N. Stereochemistry in the oxidation of primary amines to nitro compounds by dimethyldioxirane. Tetrahedron Asymm. 7, 1611–1619 (1996).
Acknowledgements
C.R.J.S. and S.M. acknowledge the financial support from the National Science Foundation (CBET-2033714), and the University of Michigan. D.A.P. acknowledges the financial support from the Natural Sciences and Engineering Research Council of Canada (RGPIN-2022-05058). C.Y. acknowledges a Rackham pre-doctoral fellowship from the University of Michigan.
Author information
Authors and Affiliations
Contributions
C.Y., S.M., D.A.P. and C.R.J.S. contemplated the topic and structure of the Review. C.Y. conducted the literature research. All authors contributed to the discussion of the content and wrote or edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Chemistry thanks Jie Wu, Osvaldo Lanzalunga, Giorgio Olivio and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, C., Arora, S., Maldonado, S. et al. The design of PINO-like hydrogen-atom-transfer catalysts. Nat Rev Chem 7, 653–666 (2023). https://doi.org/10.1038/s41570-023-00511-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-023-00511-z
This article is cited by
-
Pyridine N-oxides as hydrogen atom transfer reagents for site-selective photoinduced C(sp3)–H functionalization
Nature Synthesis (2024)
-
Reaction strategies for the meta-selective functionalization of pyridine through dearomatization
Molecular Diversity (2024)