Abstract
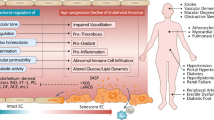
Endothelial cells are located at the crucial interface between circulating blood and semi-solid tissues and have many important roles in maintaining systemic physiological function. The vascular endothelium is particularly susceptible to pathogenic stimuli that activate tumour suppressor pathways leading to cellular senescence. We now understand that senescent endothelial cells are highly active, secretory and pro-inflammatory, and have an aberrant morphological phenotype. Moreover, endothelial senescence has been identified as an important contributor to various cardiovascular and metabolic diseases. In this Review, we discuss the consequences of endothelial cell exposure to damaging stimuli (haemodynamic forces and circulating and endothelial-derived factors) and the cellular and molecular mechanisms that induce endothelial cell senescence. We also discuss how endothelial cell senescence causes arterial dysfunction and contributes to clinical cardiovascular diseases and metabolic disorders. Finally, we summarize the latest evidence on the effect of eliminating senescent endothelial cells (senolysis) and identify important remaining questions to be addressed in future studies.
Key points
-
Forming the inner lining of blood vessels, endothelial cells are exposed to a unique milieu of damaging stimuli, including haemodynamic forces as well as circulating and endothelium-derived factors.
-
Exposure to damaging stimuli results in telomeric and non-telomeric DNA damage, mitochondrial dysfunction and alterations in energy sensor pathways in endothelial cells.
-
Changes induced by damaging stimuli lead to the activation of tumour suppressor pathways, such as p53–p21 and pRb–p16, resulting in proliferative arrest and senescence.
-
Senescent endothelial cells are enlarged, flat and refractory to changes in response to shear stress; they are also metabolically active and secrete a variety of inflammatory molecules.
-
Senescent endothelial cells and their secreted factors are major contributors to arterial dysfunction and the pathophysiology of various cardiometabolic diseases.
-
Emerging evidence suggests that targeting senescent endothelial cells can be an effective strategy to suppress cardiometabolic diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Campisi, J. & d’Adda di Fagagna, F. Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 8, 729–740 (2007).
Yousefzadeh, M. J. et al. Tissue specificity of senescent cell accumulation during physiologic and accelerated aging of mice. Aging Cell 19, e13094 (2020).
Grosse, L. et al. Defined p16(High) senescent cell types are indispensable for mouse healthspan. Cell Metab. 32, 87–99.e6 (2020).
Cohen, C. et al. Glomerular endothelial cell senescence drives age-related kidney disease through PAI-1. EMBO Mol. Med. 13, e14146 (2021).
Shosha, E. et al. Mechanisms of diabetes-induced endothelial cell senescence: role of arginase 1. Int. J. Mol. Sci. 19, 1215 (2018).
Kiss, T. et al. Single-cell RNA sequencing identifies senescent cerebromicrovascular endothelial cells in the aged mouse brain. Geroscience 42, 429–444 (2020).
Yokoi, T. et al. Apoptosis signal-regulating kinase 1 mediates cellular senescence induced by high glucose in endothelial cells. Diabetes 55, 1660–1665 (2006).
Hayashi, T. et al. Endothelial cellular senescence is inhibited by liver X receptor activation with an additional mechanism for its atheroprotection in diabetes. Proc. Natl Acad. Sci. USA 111, 1168–1173 (2014).
Gasek, N. S., Kuchel, G. A., Kirkland, J. L. & Xu, M. Strategies for targeting senescent cells in human disease. Nat. Aging 1, 870–879 (2021).
Dolgin, E. Send in the senolytics. Nat. Biotechnol. 38, 1371–1377 (2020).
Jurk, D. & Passos, J. F. Senolytic drugs: beyond the promise and the hype. Mech. Ageing Dev. 202, 111631 (2022).
Zhu, Y. et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14, 644–658 (2015).
Zhu, Y. et al. New agents that target senescent cells: the flavone, fisetin, and the BCL-X(L) inhibitors, A1331852 and A1155463. Aging 9, 955–963 (2017).
Palmer, A. K., Tchkonia, T. & Kirkland, J. L. Senolytics: potential for alleviating diabetes and its complications. Endocrinology 162, bqab058 (2021).
Raffaele, M. & Vinciguerra, M. The costs and benefits of senotherapeutics for human health. Lancet Healthy Longev. 3, e67–e77 (2022).
Wissler Gerdes, E. O., Misra, A., Netto, J. M. E., Tchkonia, T. & Kirkland, J. L. Strategies for late phase preclinical and early clinical trials of senolytics. Mech. Ageing Dev. 200, 111591 (2021).
Donato, A. J., Morgan, R. G., Walker, A. E. & Lesniewski, L. A. Cellular and molecular biology of aging endothelial cells. J. Mol. Cell Cardiol. 89, 122–135 (2015).
De Cecco, M. et al. L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature 566, 73–78 (2019).
Uryga, A. K. et al. Telomere damage promotes vascular smooth muscle cell senescence and immune cell recruitment after vessel injury. Commun. Biol. 4, 611 (2021).
Wang, B. et al. An inducible p21-Cre mouse model to monitor and manipulate p21-highly-expressing senescent cells in vivo. Nat. Aging 1, 962–973 (2021).
Wang, L. et al. Targeting p21(Cip1) highly expressing cells in adipose tissue alleviates insulin resistance in obesity. Cell Metab. 34, 75–89.e8 (2022).
d’Adda di Fagagna, F. Living on a break: cellular senescence as a DNA-damage response. Nat. Rev. Cancer 8, 512–522 (2008).
Childs, B. G., Baker, D. J., Kirkland, J. L., Campisi, J. & van Deursen, J. M. Senescence and apoptosis: dueling or complementary cell fates? EMBO Rep. 15, 1139–1153 (2014).
Hsu, C. H., Altschuler, S. J. & Wu, L. F. Patterns of early p21 dynamics determine proliferation-senescence cell fate after chemotherapy. Cell 178, 361–373.e12 (2019).
Lee, B. Y. et al. Senescence-associated β-galactosidase is lysosomal β-galactosidase. Aging Cell 5, 187–195 (2006).
Kohli, J. et al. Algorithmic assessment of cellular senescence in experimental and clinical specimens. Nat. Protoc. 16, 2471–2498 (2021).
González-Gualda, E., Baker, A. G., Fruk, L. & Muñoz-Espín, D. A guide to assessing cellular senescence in vitro and in vivo. FEBS J. 288, 56–80 (2021).
Wiley, C. D. et al. Analysis of individual cells identifies cell-to-cell variability following induction of cellular senescence. Aging Cell 16, 1043–1050 (2017).
Hernandez-Segura, A. et al. Unmasking transcriptional heterogeneity in senescent cells. Curr. Biol. 27, 2652–2660.e4 (2017).
Basisty, N. et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 18, e3000599 (2020).
Coppé, J. P., Desprez, P. Y., Krtolica, A. & Campisi, J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 5, 99–11 (2010).
Chala, N. et al. Mechanical fingerprint of senescence in endothelial cells. Nano Lett. 21, 4911–4920 (2021).
Wang, C., Baker, B. M., Chen, C. S. & Schwartz, M. A. Endothelial cell sensing of flow direction. Arterioscler. Thromb. Vasc. Biol. 33, 2130–2136 (2013).
Lafargue, A. et al. Ionizing radiation induces long-term senescence in endothelial cells through mitochondrial respiratory complex II dysfunction and superoxide generation. Free Radic. Biol. Med. 108, 750–759 (2017).
Huo, J. et al. Coenzyme Q10 prevents senescence and dysfunction caused by oxidative stress in vascular endothelial cells. Oxid. Med. Cell Longev. 2018, 3181759 (2018).
Donato, A. J. et al. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-κB. Circ. Res. 100, 1659–1666 (2007).
Khan, S. Y. et al. Premature senescence of endothelial cells upon chronic exposure to TNFα can be prevented by N-acetyl cysteine and plumericin. Sci. Rep. 7, 39501 (2017).
Yin, Y. et al. Vascular endothelial cells senescence is associated with NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome activation via reactive oxygen species (ROS)/thioredoxin-interacting protein (TXNIP) pathway. Int. J. Biochem. Cell Biol. 84, 22–34 (2017).
Bent, E. H., Gilbert, L. A. & Hemann, M. T. A senescence secretory switch mediated by PI3K/AKT/mTOR activation controls chemoprotective endothelial secretory responses. Genes Dev. 30, 1811–1821 (2016).
Hampel, B. et al. Increased expression of extracellular proteins as a hallmark of human endothelial cell in vitro senescence. Exp. Gerontol. 41, 474–481 (2006).
Hwang, H. J. et al. Endothelial cells under therapy-induced senescence secrete CXCL11, which increases aggressiveness of breast cancer cells. Cancer Lett. 490, 100–110 (2020).
Ghosh, A. K. et al. A small molecule inhibitor of PAI-1 protects against doxorubicin-induced cellular senescence. Oncotarget 7, 72443–72457 (2016).
Grillari, J., Hohenwarter, O., Grabherr, R. M. & Katinger, H. Subtractive hybridization of mRNA from early passage and senescent endothelial cells. Exp. Gerontol. 35, 187–197 (2000).
Chen, L., Holder, R., Porter, C. & Shah, Z. Vitamin D3 attenuates doxorubicin-induced senescence of human aortic endothelial cells by upregulation of IL-10 via the pAMPKα/Sirt1/Foxo3a signaling pathway. PLoS ONE 16, e0252816 (2021).
Li, R. et al. Long-term stimulation of angiotensin II induced endothelial senescence and dysfunction. Exp. Gerontol. 119, 212–220 (2019).
Shelton, D. N., Chang, E., Whittier, P. S., Choi, D. & Funk, W. D. Microarray analysis of replicative senescence. Curr. Biol. 9, 939–945 (1999).
Lee, O. H. et al. Sirtuin 6 deficiency induces endothelial cell senescence via downregulation of forkhead box M1 expression. Aging 12, 20946–20967 (2020).
Minamino, T. et al. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation 105, 1541–1544 (2002).
Coleman, P. R. et al. Stress-induced premature senescence mediated by a novel gene, SENEX, results in an anti-inflammatory phenotype in endothelial cells. Blood 116, 4016–4024 (2010).
Coleman, P. R. et al. Age-associated stresses induce an anti-inflammatory senescent phenotype in endothelial cells. Aging 5, 913–924 (2013).
Powter, E. E. et al. Caveolae control the anti-inflammatory phenotype of senescent endothelial cells. Aging Cell 14, 102–111 (2015).
Kamino, H. et al. Searching for genes involved in arteriosclerosis: proteomic analysis of cultured human umbilical vein endothelial cells undergoing replicative senescence. Cell Struct. Funct. 28, 495–503 (2003).
Bautista-Niño, P. K. et al. Local endothelial DNA repair deficiency causes aging-resembling endothelial-specific dysfunction. Clin. Sci. 134, 727–746 (2020).
Barinda, A. J. et al. Endothelial progeria induces adipose tissue senescence and impairs insulin sensitivity through senescence associated secretory phenotype. Nat. Commun. 11, 481 (2020).
Nelson, G. et al. A senescent cell bystander effect: senescence-induced senescence. Aging Cell 11, 345–349 (2012).
Erusalimsky, J. D. Vascular endothelial senescence: from mechanisms to pathophysiology. J. Appl. Physiol. 106, 326–332 (2009).
Woywodt, A., Bahlmann, F. H., De Groot, K., Haller, H. & Haubitz, M. Circulating endothelial cells: life, death, detachment and repair of the endothelial cell layer. Nephrol. Dial. Transpl. 17, 1728–1730 (2002).
Hobson, B. & Denekamp, J. Endothelial proliferation in tumours and normal tissues: continuous labelling studies. Br. J. Cancer 49, 405–413 (1984).
Chiu, J. J. & Chien, S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol. Rev. 91, 327–387 (2011).
Kotla, S. et al. Endothelial senescence is induced by phosphorylation and nuclear export of telomeric repeat binding factor 2-interacting protein. JCI Insight 4, e124867 (2019).
Warboys, C. M. et al. Disturbed flow promotes endothelial senescence via a p53-dependent pathway. Arterioscler. Thromb. Vasc. Biol. 34, 985–995 (2014).
Chang, E. & Harley, C. B. Telomere length and replicative aging in human vascular tissues. Proc. Natl Acad. Sci. USA 92, 11190–11194 (1995).
Vaupel, P., Kallinowski, F. & Okunieff, P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 49, 6449–6465 (1989).
Richardson, R. S. et al. Human skeletal muscle intracellular oxygenation: the impact of ambient oxygen availability. J. Physiol. 571, 415–424 (2006).
Chen, Q., Fischer, A., Reagan, J. D., Yan, L. J. & Ames, B. N. Oxidative DNA damage and senescence of human diploid fibroblast cells. Proc. Natl Acad. Sci. USA 92, 4337–4341 (1995).
von Zglinicki, T., Saretzki, G., Döcke, W. & Lotze, C. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Exp. Cell Res. 220, 186–193 (1995).
Parrinello, S. et al. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat. Cell Biol. 5, 741–747 (2003).
Lee, A. C. et al. Ras proteins induce senescence by altering the intracellular levels of reactive oxygen species. J. Biol. Chem. 274, 7936–7940 (1999).
Packer, L. & Fuehr, K. Low oxygen concentration extends the lifespan of cultured human diploid cells. Nature 267, 423–425 (1977).
Busuttil, R. A., Rubio, M., Dollé, M. E., Campisi, J. & Vijg, J. Oxygen accelerates the accumulation of mutations during the senescence and immortalization of murine cells in culture. Aging Cell 2, 287–294 (2003).
Basu, R. et al. Effects of age and sex on postprandial glucose metabolism: differences in glucose turnover, insulin secretion, insulin action, and hepatic insulin extraction. Diabetes 55, 2001–2014 (2006).
Issa, J. S., Diament, J. & Forti, N. Postprandial lipemia: influence of aging. Arq. Bras. Cardiol. 85, 15–19 (2005).
Lindberg, O., Tilvis, R. S. & Strandberg, T. E. Does fasting plasma insulin increase by age in the general elderly population? Aging 9, 277–280 (1997).
Trott, D. W. et al. T lymphocyte depletion ameliorates age-related metabolic impairments in mice. Geroscience 43, 1331–1347 (2021).
Prattichizzo, F. et al. Short-term sustained hyperglycaemia fosters an archetypal senescence-associated secretory phenotype in endothelial cells and macrophages. Redox Biol. 15, 170–181 (2018).
Zhong, W., Zou, G., Gu, J. & Zhang, J. L-arginine attenuates high glucose-accelerated senescence in human umbilical vein endothelial cells. Diabetes Res. Clin. Pract. 89, 38–45 (2010).
Hayashi, T. et al. Endothelial cellular senescence is inhibited by nitric oxide: implications in atherosclerosis associated with menopause and diabetes. Proc. Natl Acad. Sci. USA 103, 17018–17023 (2006).
Maeda, M., Hayashi, T., Mizuno, N., Hattori, Y. & Kuzuya, M. Intermittent high glucose implements stress-induced senescence in human vascular endothelial cells: role of superoxide production by NADPH oxidase. PLoS ONE 10, e0123169 (2015).
Mortuza, R., Chen, S., Feng, B., Sen, S. & Chakrabarti, S. High glucose induced alteration of SIRTs in endothelial cells causes rapid aging in a p300 and FOXO regulated pathway. PLoS ONE 8, e54514 (2013).
Liu, J. et al. Glucose-induced oxidative stress and accelerated aging in endothelial cells are mediated by the depletion of mitochondrial SIRTs. Physiol. Rep. 8, e14331 (2020).
Brodsky, S. V. et al. Prevention and reversal of premature endothelial cell senescence and vasculopathy in obesity-induced diabetes by ebselen. Circ. Res. 94, 377–384 (2004).
Yokoyama, M. et al. Inhibition of endothelial p53 improves metabolic abnormalities related to dietary obesity. Cell Rep. 7, 1691–1703 (2014).
Miyauchi, H. et al. Akt negatively regulates the in vitro lifespan of human endothelial cells via a p53/p21-dependent pathway. EMBO J. 23, 212–220 (2004).
Hayashi, T. et al. Nitric oxide and endothelial cellular senescence. Pharmacol. Ther. 120, 333–339 (2008).
Oh, S. T., Park, H., Yoon, H. J. & Yang, S. Y. Long-term treatment of native LDL induces senescence of cultured human endothelial cells. Oxid. Med. Cell Longev. 2017, 6487825 (2017).
Jiang, Y. H., Jiang, L. Y., Wang, Y. C., Ma, D. F. & Li, X. Quercetin attenuates atherosclerosis via modulating oxidized LDL-induced endothelial cellular senescence. Front. Pharmacol. 11, 512 (2020).
Liu, R., Cheng, F., Zeng, K., Li, W. & Lan, J. GPR120 agonist GW9508 ameliorated cellular senescence induced by ox-LDL. ACS Omega 5, 32195–32202 (2020).
Zhang, D. et al. Homocysteine accelerates senescence of endothelial cells via DNA hypomethylation of human telomerase reverse transcriptase. Arterioscler. Thromb. Vasc. Biol. 35, 71–78 (2015).
Shi, Q. et al. Endothelial senescence after high-cholesterol, high-fat diet challenge in baboons. Am. J. Physiol. Heart Circ. Physiol. 292, H2913–H2920 (2007).
Albertini, E., Kozieł, R., Dürr, A., Neuhaus, M. & Jansen-Dürr, P. Cystathionine beta synthase modulates senescence of human endothelial cells. Aging 4, 664–673 (2012).
Scalera, F. et al. Effect of L-arginine on asymmetric dimethylarginine (ADMA) or homocysteine-accelerated endothelial cell aging. Biochem. Biophys. Res. Commun. 345, 1075–1082 (2006).
Xu, D., Neville, R. & Finkel, T. Homocysteine accelerates endothelial cell senescence. FEBS Lett. 470, 20–24 (2000).
Xing, S. S. et al. Salidroside attenuates endothelial cellular senescence via decreasing the expression of inflammatory cytokines and increasing the expression of SIRT3. Mech. Ageing Dev. 175, 1–6 (2018).
Parkhitko, A. A., Jouandin, P., Mohr, S. E. & Perrimon, N. Methionine metabolism and methyltransferases in the regulation of aging and lifespan extension across species. Aging Cell 18, e13034 (2019).
Gimbrone, M. A. Jr & García-Cardeña, G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 118, 620–636 (2016).
Yamazaki, Y. et al. Vascular cell senescence contributes to blood-brain barrier breakdown. Stroke 47, 1068–1077 (2016).
Krouwer, V. J., Hekking, L. H., Langelaar-Makkinje, M., Regan-Klapisz, E. & Post, J. A. Endothelial cell senescence is associated with disrupted cell-cell junctions and increased monolayer permeability. Vasc. Cell 4, 12 (2012).
Oeseburg, H. et al. Bradykinin protects against oxidative stress-induced endothelial cell senescence. Hypertension 53, 417–422 (2009).
Vasa, M., Breitschopf, K., Zeiher, A. M. & Dimmeler, S. Nitric oxide activates telomerase and delays endothelial cell senescence. Circ. Res. 87, 540–542 (2000).
Matsushita, H. et al. eNOS activity is reduced in senescent human endothelial cells: preservation by hTERT immortalization. Circ. Res. 89, 793–798 (2001).
Olmos, G. et al. Hyperphosphatemia induces senescence in human endothelial cells by increasing endothelin-1 production. Aging Cell 16, 1300–1312 (2017).
Donato, A. J. et al. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am. J. Physiol. Heart Circ. Physiol. 297, H425–H432 (2009).
Shan, H., Bai, X. & Chen, X. Angiotensin II induces endothelial cell senescence via the activation of mitogen-activated protein kinases. Cell Biochem. Funct. 26, 459–466 (2008).
Khan, I. et al. Low dose chronic angiotensin ii induces selective senescence of kidney endothelial cells. Front. Cell Dev. Biol. 9, 782841 (2021).
Kim, M. Y. et al. The PPARδ-mediated inhibition of angiotensin II-induced premature senescence in human endothelial cells is SIRT1-dependent. Biochem. Pharmacol. 84, 1627–1634 (2012).
Kandhaya-Pillai, R. et al. TNFα-senescence initiates a STAT-dependent positive feedback loop, leading to a sustained interferon signature, DNA damage, and cytokine secretion. Aging 9, 2411–2435 (2017).
Yamagata, K., Suzuki, S. & Tagami, M. Docosahexaenoic acid prevented tumor necrosis factor alpha-induced endothelial dysfunction and senescence. Prostaglandins Leukot. Essent. Fatty Acids 104, 11–18 (2016).
Luu, A. Z. et al. Role of endothelium in doxorubicin-induced cardiomyopathy. JACC Basic Transl Sci. 3, 861–870 (2018).
Terwoord, J. D., Beyer, A. M. & Gutterman, D. D. Endothelial dysfunction as a complication of anti-cancer therapy. Pharmacol. Ther. 237, 108116 (2022).
Yeh, E. T. et al. Cardiovascular complications of cancer therapy: diagnosis, pathogenesis, and management. Circulation 109, 3122–3131 (2004).
Mongiardi, M. P. et al. Axitinib exposure triggers endothelial cells senescence through ROS accumulation and ATM activation. Oncogene 38, 5413–5424 (2019).
Clayton, Z. S. et al. Doxorubicin-induced oxidative stress and endothelial dysfunction in conduit arteries is prevented by mitochondrial-specific antioxidant treatment. JACC CardioOncol 2, 475–488 (2020).
Clayton, Z. S. et al. Tumor necrosis factor alpha-mediated inflammation and remodeling of the extracellular matrix underlies aortic stiffening induced by the common chemotherapeutic agent doxorubicin. Hypertension 77, 1581–1590 (2021).
Hutton, D. et al. Cellular senescence mediates doxorubicin-induced arterial dysfunction via activation of mitochondrial oxidative stress and the mammalian target of rapamycin [abstract]. FASEB J. https://doi.org/10.1096/fasebj.2021.35.S1.00283 (2021).
Merolle, M., Mongiardi, M. P., Piras, M., Levi, A. & Falchetti, M. L. Glioblastoma cells do not affect axitinib-dependent senescence of HUVECs in a transwell coculture model. Int. J. Mol. Sci. 21, 1490 (2020).
Wang, Y., Boerma, M. & Zhou, D. Ionizing radiation-induced endothelial cell senescence and cardiovascular diseases. Radiat. Res. 186, 153–161 (2016).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT04733534 (2022).
McDonald, A. I. et al. Endothelial regeneration of large vessels is a biphasic process driven by local cells with distinct proliferative capacities. Cell Stem Cell 23, 210–225.e6 (2018).
Hastings, R., Qureshi, M., Verma, R., Lacy, P. S. & Williams, B. Telomere attrition and accumulation of senescent cells in cultured human endothelial cells. Cell Prolif. 37, 317–324 (2004).
Roake, C. M. & Artandi, S. E. Control of cellular aging, tissue function, and cancer by p53 downstream of telomeres. Cold Spring Harb. Perspect. Med. 7, a026088 (2017).
Kurz, D. J. et al. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J. Cell Sci. 117, 2417–2426 (2004).
von Zglinicki, T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 27, 339–344 (2002).
Hewitt, G. et al. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat. Commun. 3, 708 (2012).
Oikawa, S., Tada-Oikawa, S. & Kawanishi, S. Site-specific DNA damage at the GGG sequence by UVA involves acceleration of telomere shortening. Biochemistry 40, 4763–4768 (2001).
Anderson, R. et al. Length-independent telomere damage drives post-mitotic cardiomyocyte senescence. EMBO J. 38, e100492 (2019).
Morgan, R. G., Donato, A. J. & Walker, A. E. Telomere uncapping and vascular aging. Am. J. Physiol. Heart Circ. Physiol. 315, H1–H5 (2018).
Morgan, R. G. et al. Age-related telomere uncapping is associated with cellular senescence and inflammation independent of telomere shortening in human arteries. Am. J. Physiol. Heart Circ. Physiol. 305, H251–H258 (2013).
Morgan, R. G. et al. Role of arterial telomere dysfunction in hypertension: relative contributions of telomere shortening and telomere uncapping. J. Hypertens. 32, 1293–1299 (2014).
Morgan, R. G. et al. Induced Trf2 deletion leads to aging vascular phenotype in mice associated with arterial telomere uncapping, senescence signaling, and oxidative stress. J. Mol. Cell Cardiol. 127, 74–82 (2019).
Bhayadia, R., Schmidt, B. M., Melk, A. & Hömme, M. Senescence-induced oxidative stress causes endothelial dysfunction. J. Gerontol. A Biol. Sci. Med. Sci. 71, 161–169 (2016).
Dominic, A., Banerjee, P., Hamilton, D. J., Le, N. T. & Abe, J. I. Time-dependent replicative senescence vs. disturbed flow-induced pre-mature aging in atherosclerosis. Redox Biol. 37, 101614 (2020).
Liu, Y., Bloom, S. I. & Donato, A. J. The role of senescence, telomere dysfunction and shelterin in vascular aging. Microcirculation 26, e12487 (2019).
Zhan, H., Suzuki, T., Aizawa, K., Miyagawa, K. & Nagai, R. Ataxia telangiectasia mutated (ATM)-mediated DNA damage response in oxidative stress-induced vascular endothelial cell senescence. J. Biol. Chem. 285, 29662–29670 (2010).
Kim, K. S., Kim, J. E., Choi, K. J., Bae, S. & Kim, D. H. Characterization of DNA damage-induced cellular senescence by ionizing radiation in endothelial cells. Int. J. Radiat. Biol. 90, 71–80 (2014).
Houtkooper, R. H., Pirinen, E. & Auwerx, J. Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 13, 225–238 (2012).
Liu, R., Liu, H., Ha, Y., Tilton, R. G. & Zhang, W. Oxidative stress induces endothelial cell senescence via downregulation of Sirt6. Biomed. Res. Int. 2014, 902842 (2014).
Cardus, A., Uryga, A. K., Walters, G. & Erusalimsky, J. D. SIRT6 protects human endothelial cells from DNA damage, telomere dysfunction, and senescence. Cardiovasc. Res. 97, 571–579 (2013).
Zu, Y. et al. SIRT1 promotes proliferation and prevents senescence through targeting LKB1 in primary porcine aortic endothelial cells. Circ. Res. 106, 1384–1393 (2010).
Ota, H. et al. Sirt1 modulates premature senescence-like phenotype in human endothelial cells. J. Mol. Cell Cardiol. 43, 571–579 (2007).
Yuen, L. H. et al. A focused DNA-encoded chemical library for the discovery of inhibitors of NAD+-dependent enzymes. J. Am. Chem. Soc. 141, 5169–5181 (2019).
Chen, T. et al. SIRT3 protects endothelial cells from high glucose-induced senescence and dysfunction via the p53 pathway. Life Sci. 264, 118724 (2021).
Dikalova, A. E. et al. Mitochondrial deacetylase sirt3 reduces vascular dysfunction and hypertension while Sirt3 depletion in essential hypertension is linked to vascular inflammation and oxidative stress. Circ. Res. 126, 439–452 (2020).
Correia-Melo, C. & Passos, J. F. Mitochondria: are they causal players in cellular senescence? Biochim. Biophys. Acta 1847, 1373–1379 (2015).
Wiley, C. D. et al. Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metab. 23, 303–314 (2016).
Eelen, G. et al. Endothelial cell metabolism. Physiol. Rev. 98, 3–58 (2018).
Sakamuri, S. et al. Glycolytic and oxidative phosphorylation defects precede the development of senescence in primary human brain microvascular endothelial cells. Geroscience https://doi.org/10.1007/s11357-022-00550-2 (2022).
Voghel, G. et al. Chronic treatment with N-acetyl-cystein delays cellular senescence in endothelial cells isolated from a subgroup of atherosclerotic patients. Mech. Ageing Dev. 129, 261–270 (2008).
Lener, B. et al. The NADPH oxidase Nox4 restricts the replicative lifespan of human endothelial cells. Biochem. J. 423, 363–374 (2009).
Donato, A. J., Machin, D. R. & Lesniewski, L. A. Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circ. Res. 123, 825–848 (2018).
Csiszar, A., Wang, M., Lakatta, E. G. & Ungvari, Z. Inflammation and endothelial dysfunction during aging: role of NF-κB. J. Appl. Physiol. 105, 1333–1341 (2008).
Rippe, C. et al. MicroRNA changes in human arterial endothelial cells with senescence: relation to apoptosis, eNOS and inflammation. Exp. Gerontol. 47, 45–51 (2012).
Tarantini, S. et al. Treatment with the BCL-2/BCL-xL inhibitor senolytic drug ABT263/navitoclax improves functional hyperemia in aged mice. Geroscience 43, 2427–2440 (2021).
Roos, C. M. et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 15, 973–977 (2016).
Rossman, M. J. et al. Endothelial cell senescence with aging in healthy humans: prevention by habitual exercise and relation to vascular endothelial function. Am. J. Physiol. Heart Circ. Physiol. 313, H890–H895 (2017).
Lähteenvuo, J. & Rosenzweig, A. Effects of aging on angiogenesis. Circ. Res. 110, 1252–1264 (2012).
Islam, M. T. et al. Aging differentially impacts vasodilation and angiogenesis in arteries from the white and brown adipose tissues. Exp. Gerontol. 142, 111126 (2020).
El Maï, M. et al. The telomeric protein TRF2 regulates angiogenesis by binding and activating the PDGFRβ promoter. Cell Rep. 9, 1047–1060 (2014).
Franco, S., Segura, I., Riese, H. H. & Blasco, M. A. Decreased B16F10 melanoma growth and impaired vascularization in telomerase-deficient mice with critically short telomeres. Cancer Res. 62, 552–559 (2002).
Zaccagnini, G. et al. Telomerase mediates vascular endothelial growth factor-dependent responsiveness in a rat model of hind limb ischemia. J. Biol. Chem. 280, 14790–14798 (2005).
Ungvari, Z. et al. Ionizing radiation promotes the acquisition of a senescence-associated secretory phenotype and impairs angiogenic capacity in cerebromicrovascular endothelial cells: role of increased DNA damage and decreased DNA repair capacity in microvascular radiosensitivity. J. Gerontol. A Biol. Sci. Med. Sci. 68, 1443–1457 (2013).
Yoshida, Y. et al. p53-Induced inflammation exacerbates cardiac dysfunction during pressure overload. J. Mol. Cell Cardiol. 85, 183–198 (2015).
Chang, H. et al. Telomerase- and angiogenesis-related gene responses to irradiation in human umbilical vein endothelial cells. Int. J. Mol. Med. 31, 1202–1208 (2013).
Brühl, T. et al. p21Cip1 levels differentially regulate turnover of mature endothelial cells, endothelial progenitor cells, and in vivo neovascularization. Circ. Res. 94, 686–692 (2004).
Gogiraju, R. et al. Endothelial p53 deletion improves angiogenesis and prevents cardiac fibrosis and heart failure induced by pressure overload in mice. J. Am. Heart Assoc. 4, e001770 (2015).
Gu, J. et al. Inhibition of p53 prevents diabetic cardiomyopathy by preventing early-stage apoptosis and cell senescence, reduced glycolysis, and impaired angiogenesis. Cell Death Dis. 9, 82 (2018).
Akimoto, S., Mitsumata, M., Sasaguri, T. & Yoshida, Y. Laminar shear stress inhibits vascular endothelial cell proliferation by inducing cyclin-dependent kinase inhibitor p21(Sdi1/Cip1/Waf1). Circ. Res. 86, 185–190 (2000).
Crespo-Garcia, S. et al. Pathological angiogenesis in retinopathy engages cellular senescence and is amenable to therapeutic elimination via BCL-xL inhibition. Cell Metab. 33, 818–832.e7 (2021).
Claesson-Welsh, L., Dejana, E. & McDonald, D. M. Permeability of the endothelial barrier: identifying and reconciling controversies. Trends Mol. Med. 27, 314–331 (2021).
Salvador, E. et al. Senescence and associated blood-brain barrier alterations in vitro. Histochem. Cell Biol. 156, 283–292 (2021).
Buford, T. W. Hypertension and aging. Ageing Res. Rev. 26, 96–111 (2016).
Voghel, G. et al. Cellular senescence in endothelial cells from atherosclerotic patients is accelerated by oxidative stress associated with cardiovascular risk factors. Mech. Ageing Dev. 128, 662–671 (2007).
Westhoff, J. H. et al. Hypertension induces somatic cellular senescence in rats and humans by induction of cell cycle inhibitor p16INK4a. Hypertension 52, 123–129 (2008).
McCarthy, C. G., Wenceslau, C. F., Webb, R. C. & Joe, B. Novel contributors and mechanisms of cellular senescence in hypertension-associated premature vascular aging. Am. J. Hypertens. 32, 709–719 (2019).
Pérez-Rivero, G. et al. Mice deficient in telomerase activity develop hypertension because of an excess of endothelin production. Circulation 114, 309–317 (2006).
de Montgolfier, O. et al. High systolic blood pressure induces cerebral microvascular endothelial dysfunction, neurovascular unit damage, and cognitive decline in mice. Hypertension 73, 217–228 (2019).
Islam, T. Impact of statins on vascular smooth muscle cells and relevance to atherosclerosis. J. Physiol. 598, 2295–2296 (2020).
Bentzon, J. F., Otsuka, F., Virmani, R. & Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 114, 1852–1866 (2014).
Childs, B. G. et al. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 354, 472–477 (2016).
Honda, S. et al. Cellular senescence promotes endothelial activation through epigenetic alteration, and consequently accelerates atherosclerosis. Sci. Rep. 11, 14608 (2021).
Yanaka, M. et al. Increased monocytic adhesion by senescence in human umbilical vein endothelial cells. Biosci. Biotechnol. Biochem. 75, 1098–1103 (2011).
Silva, G. C. et al. Replicative senescence promotes prothrombotic responses in endothelial cells: role of NADPH oxidase- and cyclooxygenase-derived oxidative stress. Exp. Gerontol. 93, 7–15 (2017).
Bochenek, M. L., Schütz, E. & Schäfer, K. Endothelial cell senescence and thrombosis: ageing clots. Thromb. Res. 147, 36–45 (2016).
Tsihlis, N. D. et al. Nitric oxide inhibits vascular smooth muscle cell proliferation and neointimal hyperplasia by increasing the ubiquitination and degradation of UbcH10. Cell Biochem. Biophys. 60, 89–97 (2011).
Liu, Z. J. et al. Notch activation induces endothelial cell senescence and pro-inflammatory response: implication of Notch signaling in atherosclerosis. Atherosclerosis 225, 296–303 (2012).
Zhu, Y. et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 15, 428–435 (2016).
Caland, L. et al. Knockdown of angiopoietin-like 2 induces clearance of vascular endothelial senescent cells by apoptosis, promotes endothelial repair and slows atherogenesis in mice. Aging 11, 3832–3850 (2019).
Bai, B. et al. Cyclin-dependent kinase 5-mediated hyperphosphorylation of sirtuin-1 contributes to the development of endothelial senescence and atherosclerosis. Circulation 126, 729–740 (2012).
Dou, F. et al. PPARα targeting GDF11 inhibits vascular endothelial cell senescence in an atherosclerosis model. Oxid. Med. Cell Longev. 2021, 2045259 (2021).
Borlaug, B. A. & Redfield, M. M. Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation 123, 2006–2013 (2011).
Paulus, W. J. & Tschöpe, C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 62, 263–271 (2013).
Gevaert, A. B. et al. Endothelial senescence contributes to heart failure with preserved ejection fraction in an aging mouse model. Circ. Heart Fail. 10, e003806 (2017).
Shah, S. J. et al. Phenotype-specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation 134, 73–90 (2016).
Owens, W. A., Walaszczyk, A., Spyridopoulos, I., Dookun, E. & Richardson, G. D. Senescence and senolytics in cardiovascular disease: promise and potential pitfalls. Mech. Ageing Dev. 198, 111540 (2021).
Riehle, C. & Bauersachs, J. Small animal models of heart failure. Cardiovasc. Res. 115, 1838–1849 (2019).
Dookun, E. et al. Clearance of senescent cells during cardiac ischemia-reperfusion injury improves recovery. Aging Cell 19, e13249 (2020).
Childs, B. G., Li, H. & van Deursen, J. M. Senescent cells: a therapeutic target for cardiovascular disease. J. Clin. Invest. 128, 1217–1228 (2018).
Lawrie, A. & Francis, S. E. Frataxin and endothelial cell senescence in pulmonary hypertension. J. Clin. Invest. 131, e149721 (2021).
van der Feen, D. E. et al. Cellular senescence impairs the reversibility of pulmonary arterial hypertension. Sci. Transl Med. 12, eaaw4974 (2020).
Culley, M. K. et al. Frataxin deficiency promotes endothelial senescence in pulmonary hypertension. J. Clin. Invest. 131, e136459 (2021).
Graupera, M. & Claret, M. Endothelial cells: new players in obesity and related metabolic disorders. Trends Endocrinol. Metab. 29, 781–794 (2018).
Hasegawa, Y. et al. Blockade of the nuclear factor-κB pathway in the endothelium prevents insulin resistance and prolongs life spans. Circulation 125, 1122–1133 (2012).
Garrido, A. M. et al. Efficacy and limitations of senolysis in atherosclerosis. Cardiovasc. Res. 118, 1713–1727 (2022).
Zhu, F. et al. Senescent cardiac fibroblast is critical for cardiac fibrosis after myocardial infarction. PLoS ONE 8, e74535 (2013).
Meyer, K., Hodwin, B., Ramanujam, D., Engelhardt, S. & Sarikas, A. Essential role for premature senescence of myofibroblasts in myocardial fibrosis. J. Am. Coll. Cardiol. 67, 2018–2028 (2016).
Acknowledgements
The authors are supported by funding from National Institute of Health Awards R01 AG060395 (A.J.D.), R01 AG050238 (A.J.D.), R01 AG048366 (L.A.L.), F31AG076312 (S.I.B.) and Veteran’s Affairs Merit Review Award I01 BX004492 (L.A.L.) from the United States Department of Veterans Affairs Biomedical Laboratory Research and Development Service. The contents of the Review do not represent the views of the United States Department of Veterans Affairs, the National Institutes of Health or the United States Government.
Author information
Authors and Affiliations
Contributions
S.I.B. and M.T.I. researched data for the article. All the authors contributed substantially to discussion of the content, wrote the article, and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
A.J.D. is a scientific adviser and stockholder and L.A.L. is a stockholder in Recursion Pharmaceuticals. None of the work done with Recursion is outlined or discussed in this Review. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks Jorge Erusalimsky and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bloom, S.I., Islam, M.T., Lesniewski, L.A. et al. Mechanisms and consequences of endothelial cell senescence. Nat Rev Cardiol 20, 38–51 (2023). https://doi.org/10.1038/s41569-022-00739-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-022-00739-0
This article is cited by
-
A single-cell atlas of the aging mouse ovary
Nature Aging (2024)
-
Kdm6a-CNN1 axis orchestrates epigenetic control of trauma-induced spinal cord microvascular endothelial cell senescence to balance neuroinflammation for improved neurological repair
Bone Research (2024)
-
Cell senescence in liver diseases: pathological mechanism and theranostic opportunity
Nature Reviews Gastroenterology & Hepatology (2024)
-
MicroRNAs regulate the vicious cycle of vascular calcification-osteoporosis in postmenopausal women
Molecular Biology Reports (2024)
-
ROS-Induced Gingival Fibroblast Senescence: Implications in Exacerbating Inflammatory Responses in Periodontal Disease
Inflammation (2024)