Abstract
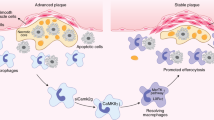
Atherosclerosis is the process that underlies heart attack and stroke. A characteristic feature of the atherosclerotic plaque is the accumulation of apoptotic cells in the necrotic core. Prophagocytic antibody-based therapies are currently being explored to stimulate the phagocytic clearance of apoptotic cells; however, these therapies can cause off-target clearance of healthy tissues, which leads to toxicities such as anaemia. Here we developed a macrophage-specific nanotherapy based on single-walled carbon nanotubes loaded with a chemical inhibitor of the antiphagocytic CD47-SIRPα signalling axis. We demonstrate that these single-walled carbon nanotubes accumulate within the atherosclerotic plaque, reactivate lesional phagocytosis and reduce the plaque burden in atheroprone apolipoprotein-E-deficient mice without compromising safety, and thereby overcome a key translational barrier for this class of drugs. Single-cell RNA sequencing analysis reveals that prophagocytic single-walled carbon nanotubes decrease the expression of inflammatory genes linked to cytokine and chemokine pathways in lesional macrophages, which demonstrates the potential of ‘Trojan horse’ nanoparticles to prevent atherosclerotic cardiovascular disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
Code used on R package for analysis of scRNA-seq data can be accessed by contacting A.M.F. at atflores@stanford.edu.
References
Arandjelovic, S. & Ravichandran, K. S. Phagocytosis of apoptotic cells in homeostasis. Nat. Immunol. 16, 907–917 (2015).
Huynh, M. L., Fadok, V. A. & Henson, P. M. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-β1 secretion and the resolution of inflammation. J. Clin. Invest. 109, 41–50 (2002).
Yurdagul, A. Jr., Doran, A. C., Cai, B., Fredman, G. & Tabas, I. A. Mechanisms and consequences of defective efferocytosis in atherosclerosis. Front. Cardiovasc. Med. 4, 86 (2017).
Jaiswal, S. et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 138, 271–285 (2009).
Willingham, S. B. et al. The CD47-signal regulatory protein alpha (SIRPα) interaction is a therapeutic target for human solid tumors. Proc. Natl Acad. Sci. USA 109, 6662–6667 (2012).
Kojima, Y. et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature 536, 86–90 (2016).
Leading Causes of Death, 1900–1998 (Centre for Disease Control and Prevention, 2015); https://www.cdc.gov/nchs/data/dvs/lead1900_98.pdf
Heron, M. & Anderson, R. N. Changes in the Leading Cause of Death: Recent Patterns in Heart Disease and Cancer Mortality (National Center for Health Statistics, 2016); https://www.cdc.gov/nchs/data/databriefs/db254.pdf
Brown, E. J. & Frazier, W. A. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 11, 130–135 (2001).
Gresham, H. D. et al. Negative regulation of phagocytosis in murine macrophages by the Src kinase family member, Fgr. J. Exp. Med. 191, 515–528 (2000).
Liu, J. et al. Pre-clinical development of a humanized Anti-CD47 antibody with anti-cancer therapeutic potential. PLoS ONE 10, e0137345 (2015).
Weiskopf, K. et al. Engineered SIRPα variants as immunotherapeutic adjuvants to anticancer antibodies. Science 341, 88–91 (2013).
Liu, Z., Sun, X., Nakayama-Ratchford, N. & Dai, H. Supramolecular chemistry on water-soluble carbon nanotubes for drug loading and delivery. ACS Nano 1, 50–56 (2007).
Schipper, M. L. et al. A pilot toxicology study of single-walled carbon nanotubes in a small sample of mice. Nat. Nanotechnol. 3, 216–221 (2008).
Liu, Z. et al. Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by Raman spectroscopy. Proc. Natl Acad. Sci. USA 105, 1410–1415 (2008).
Smith, B. R. et al. Selective uptake of single-walled carbon nanotubes by circulating monocytes for enhanced tumour delivery. Nat. Nanotechnol. 9, 481–487 (2014).
Swirski, F. K. et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J. Clin. Invest. 117, 195–205 (2007).
Robbins, C. S. et al. Extramedullary hematopoiesis generates Ly-6Chigh monocytes that infiltrate atherosclerotic lesions. Circulation 125, 364–374 (2012).
Swirski, F. K. et al. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc. Natl Acad. Sci. USA 103, 10340–10345 (2006).
Moore, K. J. & Tabas, I. Macrophages in the pathogenesis of atherosclerosis. Cell 145, 341–355 (2011).
Liu, Z. et al. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nat. Nanotechnol. 2, 47–52 (2007).
Campagnolo, L. et al. Biodistribution and toxicity of pegylated single wall carbon nanotubes in pregnant mice. Part. Fibre Toxicol. 10, 21 (2013).
Hung, S, C., Zhu, S., Ma, Z., Ghosen, E. & Mellins, E. D. Single-walled carbon nanotubes target neutrophils and Ly-6Chi monocytes and localize to joints in murine models of arthritis. J. Immunol. 175 (Suppl.), 23 (2018).
Daugherty, A., Manning, M. W. & Cassis, L. A. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J. Clin. Invest. 105, 1605–1612 (2000).
Zhang, Z., Shen, K., Lu, W. & Cole, P. A. The role of C-terminal tyrosine phosphorylation in the regulation of SHP-1 explored via expressed protein ligation. J. Biol. Chem. 278, 4668–4674 (2003).
Schrijvers, D. M., De Meyer, G. R., Kockx, M. M., Herman, A. G. & Martinet, W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 25, 1256–1261 (2005).
Poon, I. K., Lucas, C. D., Rossi, A. G. & Ravichandran, K. S. Apoptotic cell clearance: basic biology and therapeutic potential. Nat. Rev. Immunol. 14, 166–180 (2014).
Rudd, J. H. et al. Imaging atherosclerotic plaque inflammation by fluorodeoxyglucose with positron emission tomography: ready for prime time? J. Am. Coll. Cardiol. 55, 2527–2535 (2010).
Luo, G. et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 386, 78–81 (1997).
Carballo, E., Gilkeson, G. S. & Blackshear, P. J. Bone marrow transplantation reproduces the tristetraprolin-deficiency syndrome in recombination activating gene-2 (–/–) mice. Evidence that monocyte/macrophage progenitors may be responsible for TNFalpha overproduction. J. Clin. Invest. 100, 986–995 (1997).
Yoshimura, A., Naka, T. & Kubo, M. SOCS proteins, cytokine signalling and immune regulation. Nat. Rev. Immunol. 7, 454–465 (2007).
Barker, R. N. et al. Antigen presentation by macrophages is enhanced by the uptake of necrotic, but not apoptotic, cells. Clin. Exp. Immunol. 127, 220–225 (2002).
George, T. I. Automated Hematology Instrumentation (UptoDate Inc., 2018).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Advani, R. et al. CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin’s lymphoma. N. Engl. J. Med. 379, 1711–1721 (2018).
Plutzky, J., Neel, B. G. & Rosenberg, R. D. Isolation of a src homology 2-containing tyrosine phosphatase. Proc. Natl Acad. Sci. USA 89, 1123–1127 (1992).
Green, M. C. & Shultz, L. D. Motheaten, an immunodeficient mutant of the mouse. I. Genetics and pathology. J. Hered. 66, 250–258 (1975).
Kojima, Y. et al. Cyclin-dependent kinase inhibitor 2B regulates efferocytosis and atherosclerosis. J. Clin. Invest. 124, 1083–1097 (2014).
Smith, B. R. & Gambhir, S. S. Nanomaterials for in vivo imaging. Chem. Rev. 117, 901–986 (2017).
Flores, A. M. et al. Nanoparticle therapy for vascular diseases. Arterioscler. Thromb. Vasc. Biol. 39, 635–646 (2019).
Alidori, S. et al. Targeted fibrillar nanocarbon RNAi treatment of acute kidney injury. Sci. Transl. Med. 8, 331ra339 (2016).
Kagan, V. E. et al. Carbon nanotubes degraded by neutrophil myeloperoxidase induce less pulmonary inflammation. Nat. Nanotechnol. 5, 354–359 (2010).
Elgrabli, D. et al. Carbon nanotube degradation in macrophages: live nanoscale monitoring and understanding of biological pathway. ACS Nano 9, 10113–10124 (2015).
Green, D. R., Oguin, T. H. & Martinez, J. The clearance of dying cells: table for two. Cell Death Differ. 23, 915–926 (2016).
Fredman, G. et al. Targeted nanoparticles containing the proresolving peptide Ac2-26 protect against advanced atherosclerosis in hypercholesterolemic mice. Sci. Transl. Med. 7, 275ra220 (2015).
Liu, Z., Tabakman, S. M., Chen, Z. & Dai, H. Preparation of carbon nanotube bioconjugates for biomedical applications. Nat. Protocols 4, 1372–1382 (2009).
Smith, B. R. et al. Shape matters: intravital microscopy reveals surprising geometrical dependence for nanoparticles in tumor models of extravasation. Nano Lett. 12, 3369–3377 (2012).
Zheng, G. X. et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun. 8, 14049 (2017).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
Ashburner, M. et al. Gene ontology: tool for the unification of biology. The gene ontology consortium. Nat. Genet. 25, 25–29 (2000).
Acknowledgements
A.M.F. is a Howard Hughes Medical Institute Research Fellow. K.-U.J. and P.T. are supported by Deutsche Forschungsgemeinschaft grant JA 2869/1-1:1 and TS 385/1-1. This study was funded by NIH R35 HL144475, R01 HL123370, a 2018 Fondation Leducq Award (to N.J.L.), K99 CA160764 (to B.R.S.), a Catalyst Award from the Dr. Ralph and Marian Falk Medical Research Trust, the Johnson Family Foundation (to N.J.L.) and the American Heart Association Transformational Project grant 18TPA34230113 (to B.R.S. and N.J.L.). We acknowledge the Stanford Small Animal Imaging Facility, the Stanford Cyclotron & Radiochemistry Facility, the Stanford Nano Shared Facilities/Stanford Nanofabrication Facility (National Science Foundation award ECCS-1542152) and the Stanford Shared FACS facility used for sorting (NIH S10 S10RR025518-01). The sequencing data were generated on an Illumina HiSeq 4000 that was purchased with funds from NIH S10OD018220. The authors acknowledge S. Gustafsson for help with bioinformatics analyses, I. Kalashnikova for technical assistance in nanocharacterization, T. Shaffer for radiochemistry assistance, D.-H. Chen for help with the TEM imaging, M. Weglarz of the Stanford FACS facility for technical support, D. Wagh of the Stanford Functional Genomics Facility for assistance with scRNA-seq and T. Doyle for valuable input on small animal imaging.
Author information
Authors and Affiliations
Contributions
B.R.S. and N.J.L. conceived the study. A.M.F. designed and conducted the majority of experiments. N.H.-N., K.-U.J., M.L. and B.R.S. developed, produced and characterized SWNT-SHP1i. A.M.F., K.-U.J. and J.Y. performed the mouse microsurgery and histological analyses. A.M.F., N.H.-N., K.-U.J. and J.Y. conducted the flow cytometric and radiochemical biodistribution studies. K.-U.J. conducted the PET/CT imaging. X.Z. and B.R.S. performed the high-dimensional flow cytometry studies with assistance from A.M.F. and J.Y. N.H.-N, A.L.K., Y.Z., R.S. and B.R.S. performed and analysed the TEM. A.M.F. performed the scRNA-seq analysis with assistance from R.W. P.T., Y.W., V.N., Y.K., E.I. and I.L.W. contributed to experimental design and data interpretation. A.M.F., N.H.-N., K.-U.J., B.R.S. and N.J.L. wrote the manuscript. All the authors discussed the results and provided feedback on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
N.J.L. and I.L.W. are co-founders and hold equity interest in 47 Incorporated.
Additional information
Peer review information Nature Nanotechnology thanks Gabrielle Fredman, Willem Mulder and Edward Thorp for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Extended Data Fig. 1.
a, Schematic illustrating steps of SWNT-SHP1i preparation. Following SWNT-PEG-Cy5.5 (SWNT-Cy5.5) fabrication, SHP1i is loaded onto SWNT-Cy5.5 by adding SHP1i to a stirred solution of SWNT-Cy5.5 overnight at 4 °C and removing free SHP1i molecules by dialyzing with PBS for 24h at 4 °C. DSPE-PEG: 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)]. b, Chemical structure of the small-molecule inhibitor of SHP-1. c, Release rates of SHP1i from SWNT-Cy5.5 in PBS (pH=7.4). d, Of the various PEG lengths tested, PEG5000 provided the highest yield (3.5x higher than PEG2000). SWNTs were thus functionalized with PEG5000 for in vitro and in vivo studies. e-f, Photos depicting color change in the SWNT-Cy5.5 filtrate demonstrating removal of excess Cy5.5 (blue color) after each washing step (e), and after loading SWNT-Cy5.5 with SHP1i (red-tinted solution on right) (f). g, Attenuated total reflectance (ATR) infrared spectra for SWNT-SHP1i, SWNT-Cy5.5, and SHP1i. The major spectral features of SHP1i are located in the fingerprint region, containing a complex set of absorptions. The S-O stretch from SO3- in the SHP1i molecule observed at 1034 cm-1 in SHP1i spectra is recapitulated in the SWNT-SHP1i spectrum as an additional spike at 1034cm-1 in comparison with the SWNT-Cy5.5 spectrum (highlighted in square). These data confirm loading of SHP1i on SWNTs together with UV-vis spectra and the color change of the solution. Data in c-g were repeated 3 times with similar results. h, Quantification of endotoxin levels reveals that SWNT-PEG, SWNT-Cy5.5, and SWNT-SHP1i each have endotoxin levels <0.01 ng/mL (approximately 0.1 endotoxin units per mL; standard curve provided). The assay was performed once with 3 biological replicates. Mean and standard error of the mean (s.e.m.) are shown.
Extended Data Fig. 2 Extended Data Fig. 2.
a-c, In vitro uptake studies show that SWNTs are preferentially taken up by human and mouse macrophages after 3hr incubation. Uptake studies are shown in human macrophages (PMA-differentiated THP-1 cells), human aortic endothelial cells (HAECs), and human coronary artery smooth muscle cells (HCASMCs) (a-b, n = 3), as well as murine macrophages (RAW264.7), endothelial cells (C166), and primary aortic vascular smooth muscle cells (VSMCs) (c, n = minimum 3 per cell type). **p < 0.01, ****p < 0.0001 by one-way ANOVA with a Tukey post-hoc test. d, SWNTs are taken up by ~100% of basal (M0) and IL-4-polarized (M2) RAW264.7 macrophages, and ~85% of macrophages skewed towards the M1 state with LPS and IFN-γ (n = 3). ****p < 0.0001 by one-way ANOVA with a Tukey post-hoc test. e, The phagocytosis efficiency of macrophages (CellTracker Red+) against apoptotic vascular cells (CellTracker Orange+) is enhanced by SWNT-SHP1i nanoparticles, relative to SWNTs, SWNT-Cy5.5 and SHP1i controls (n = 5). *p < 0.05 by unpaired two-tailed t-test. **p < 0.01, ***p < 0.001 by one-way ANOVA with a Tukey post-hoc test. f, Representative flow cytometry plots and staining controls for the conditions of the in vitro phagocytosis assays. Double-positive cells in the right upper quadrant represent macrophages that have ingested a target apoptotic cell. g, SWNT-SHP1i treatment does not alter the rates of programmed cell death of RAW264.7 macrophages in vitro, as shown by the lack of a difference in TUNEL (terminal deoxynucleotidyl transferase [TdT] dUTP nick-end labeling) staining (n = 2). h, MTT assays show that SWNT-SHP1i has no effect on the proliferation rates of RAW264.7 macrophages in the presence of 10% serum (n = 3). i, Cell viability assays indicate that SWNTs do not affect the viability of RAW264.7 cells, suggesting the absence of a toxic effect on macrophages (n = 3). PBS served as control. Data in f are representative of 5 independent experiments. For all graphs, data are expressed as the mean and s.e.m.
Extended Data Fig. 3 Extended Data Fig. 3.
a, Assessment of serum stability of 89Zr-radiolabeled SWNTs demonstrates no signs of instability for up to 7 days at 37 °C in fresh mouse serum. Data are representative of 3 independent experiments. b, Fluorescence-based studies show that the blood half-life (t1/2) of SWNT-Cy5.5 measures ~2hr, indicating that desferrioxamine chelation of 89Zr to SWNT-Cy5.5 does not significantly alter the circulation time of the nanoparticles used in the formal biodistribution studies (n = minimum 4 biologically independent animals per time point). Mean and s.e.m. are shown. c, Representation flow cytometry plots and gating strategy for analysis of SWNT uptake in homogenized organs. d, Immunofluorescence imaging shows SWNT (immunostained for PEG) accumulation in the aortic sinus, with lesser amounts in the spleen and liver, and little-to-no accumulation in other organs such as healthy aorta, lung, and kidney after 4 weeks of weekly serial injections. Data are representative of a minimum of 3 independent experiments. Scale bars, 100 μm.
Extended Data Fig. 4 Extended Data Fig. 4.
a, Representative flow cytometry plots from in vivo cellular uptake studies after 4 weeks of serial injections show significant SWNT accumulation in atherosclerotic Ly-6Chi monocytes and macrophages, but low uptake by other vascular cells (n = 4 biologically independent animals). b, Additional confocal images demonstrate co-localization (indicated by arrows) of SWNTs (green) with macrophages (red) in the atherosclerotic aortic sinus. Macrophages were identified by immunostaining for both CD68 (top) and Mac-3 (bottom). Data are representative of 4 independent experiments. Scale bars, 50 μm.
Extended Data Fig. 5 Extended Data Fig. 5.
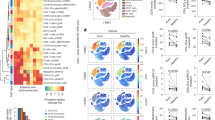
a, Study timeline detailing the “angiotensin infusion” (which includes 4 weeks of high-fat diet and weekly SWNT injections) and “chronic atherosclerosis” models (which includes 2 weeks of high-fat diet, followed by 9 weeks of SWNT treatment without angiotensin II infusion). The beneficial effect of pro-efferocytic SWNT-SHP1i was confirmed in both models of vascular disease. b, In the main angiotensin infusion model, histological analysis of lesions in the aortic sinus area show that SWNT-SHP1i results in a significant reduction in plaque area in both male (n = 9 biologically independent animals for control group, n = 10 biologically independent animals for SWNT-SHP1i group) and female mice (n = 8 biologically independent animals for control group, n = 9 biologically independent animals for SWNT-SHP1i group), as measured by Oil Red O (ORO) staining. This finding is particularly important given the widely reported sex-dependent effects on atherosclerosis mouse models that is also relevant to human disease (Supplementary ref. 1). *p < 0.05, **p < 0.01 by unpaired two-tailed t-test. c, Similar therapeutic efficacy was observed in the chronic atherosclerosis models (n = 12 biologically independent animals for control group, n = 11 biologically independent animals for SWNT-SHP1i group). *p < 0.05 by unpaired two-tailed t-test. Scale bar, 250 μm. d-f, The benefits of pro-efferocytic SWNT-SHP1i on atherosclerosis occur independently of blood pressure (n = 6 biologically independent animals per group) (d), glucose (n = 14 biologically independent animals for control group, n = 17 biologically independent animals for SWNT-SHP1i group) (e), and cholesterol levels (n = 11 biologically independent animals for control group, n = 10 biologically independent animals for SWNT-SHP1i group) (f). Blue graphs indicate results from the angiotensin infusion model, while red graphs indicated results from the chronic atherosclerosis studies. For all graphs, data are expressed as the mean and s.e.m.
Extended Data Fig. 6 Extended Data Fig. 6.
Additional histological analyses confirm that SWNT-SHP1i induces a plaque-stabilizing phenotype. a, Masson Trichrome staining indicates that SWNT-SHP1i reduces the necrotic core in both male (n = 8 biologically independent animals per group) and female mice (n = 8 biologically independent animals per group). *p < 0.05 by two-sided Mann-Whitney U test in left panel, by unpaired two-tailed t-test in right panel. Scale bar, 250 μm. b, A trend towards increased collagen content was also observed after treatment (n = 8 biologically independent animals per group). c, Additional examples of lesion tracing and necrotic core analyses indicating reduced accumulation of apoptotic and necrotic debris after treatment. Data are representative of 16 independent experiments. Scale bar, 250 μm. d, α-SMA staining indicates enhanced smooth muscle cell content in the cap, suggesting a reduction in plaque vulnerability after therapy (n = 8 biologically independent animals for control group, n = 9 biologically independent animals for SWNT-SHP1i group). *p < 0.05 by unpaired two-tailed t-test. Scale bar, 250μm. e, Additional examples of lesional caspase staining (indicated with stars) highlighting a reduction in apoptotic cell content in treated animals. Data are representative of 9 independent experiments. Scale bar, 50 μm. For all graphs, data are expressed as the mean and s.e.m.
Extended Data Fig. 7 Extended Data Fig. 7.
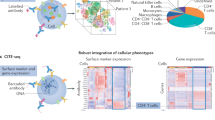
a, Flow cytometry gating strategy for selection of viable (SYTOX Blue-) cells that had taken up SWNTs (Cy5.5+). b, Sequencing data quality metrics for cells isolated from aortae of mice following treatment with SWNT-Cy5.5 or SWNT-SHP1i. c, Violin plots showing number of genes (nGene), unique molecular identifier (nUMI), and percentage of mitochondrial gene reads (percent.mito) for cells in the full dataset (n = 8 biologically independent animals). Each point represents the given value from a single cell. d, Scatterplot of nGene and nUMI across the combined dataset used to identify and exclude outliers (for example cell doublets). e, Representative violin plots showing the distribution of gene expression of immune cell markers in the 7 identified leukocyte clusters (n = 8 biologically independent animals). The identity of clusters was defined according to canonical hematopoietic-lineage and immune cell markers: macrophages (Adgre1 encoding F4/80, Cd68, Csf1r), memory T cells (Cd3g, Il2r, Ptprc and Il7r encoding memory markers CD45RO and CD127), dendritic cells (Cd209a, Flt3, Itgax encoding CD11c), monocytes (Ccr2, Ly6c2, Itgam encoding CD11b), granulocytes (Csf3r, S100a9), and CD4+/CD8+ T cell subsets (Cd3e, Cd4, Cd8a). Each point represents log-normalized single cell expression levels. f, Analysis of SWNT-positive (Cy5.5+) cells in each cluster confirms that SWNTs specifically target macrophages in the atherosclerotic aorta. Detection of SWNT uptake was greater in macrophages when characterizing cells by their whole-transcriptome, rather than the traditional limited markers used in flow cytometry above (Fig. 2e, Supplementary Fig. 4). ~90% of lesional macrophages took up SWNTs in both SWNT-Cy5.5 and SWNT-SHP1i treated animals as compared to <30% of dendritic cells and <10% of T cells and granulocytes with SWNT detection. Similarly high SWNT uptake (>75%) was detected in “macrophage-like” cells.
Extended Data Fig. 8 Extended Data Fig. 8.
a, Survival analysis indicate no change in mortality with SWNT-SHP1i treatment (n = 34 biologically independent animals for control group, n = 36 biologically independent animals for SWNT-SHP1i group). b-d, The in vivo safety of pro-efferocytic SWNTs is further supported by the stable body weight in apoE-/- mice treated with SWNT-SHP1i compared to SWNT-Cy5.5 controls (n = 22 biologically independent animals per group). e,f. Similarly, there were no differences in the weight of any organ between groups (n = 22 biologically independent animals per group). g-j, SWNT-SHP1i does not induce any major hematopoietic toxicities, such as the reduction in the red blood cell (RBC) count that is observed in anti-CD47 antibody treated mice (g). Procalcitonin levels are also unchanged between treatment groups, indicating a low likelihood for increased bacterial infections in SWNT-SHP1i-treated mice (h). There is also no effect of SWNT-SHP1i on total leukocytes, neutrophils, or monocytes (n = 18 biologically independent animals for control group, n = 22 biologically independent animals for SWNT-SHP1i group) (i). Lastly, without inducing immunosuppression, SWNT-SHP1i reduced hs-CRP levels, suggesting reduced inflammation after treatment (n = 10 biologically independent animals per group). *p = 0.03 by two-sided Mann-Whitney U test (j). Blue graphs indicate results from the angiotensin infusion model, while red graphs indicate results from the chronic atherosclerosis studies. Data from anti-CD47 and IgG-treated mice in (g) are previously reported (n = 11 biologically independent animals per group), see Supplementary ref. 2. For all graphs, data are expressed as the mean and s.e.m.
Extended Data Fig. 9 Extended Data Fig. 9.
a, Hematology assessment demonstrate that SWNT-SHP1i treatment results in a significant decrease in the mean platelet volume (MPV) and platelet-large cell ratio (P-LCR), but has no effect on major parameters of the complete blood count or metabolic panel that would indicate organ or hematopoietic toxicity (n = minimum 9 biologically independent animals per group). *p < 0.05 by unpaired two-tailed t-test. b-d, In contrast to prior publications indicating that global knockout of SHP-1 can have a variety of harmful effects including dermatitis, pneumonitis, and renal complement deposition (images adapted from Supplementary refs. 3,4,5), we observed none of these toxicities in mice treated with macrophage-specific SWNT-SHP1i6. This favorable safety profile was evidenced by a lack of hair loss or suppurative skin lesions (b), an absence of pulmonary inflammation and alveolar hemorrhage (c), and the absence of elevated renal C3 immunofluorescence staining (n = 5 per group, 4 sections analyzed per animal) (d). Scale bar in c, 100μm. Scale bars in d, 200 μm and 50 μm (insets). SHP1-deficient image in 9c reprinted with permission from Supplementary ref. 4; Copyright (2008) National Academy of Sciences, U.S.A. For all graphs, data are expressed as the mean and s.e.m.
Supplementary information
Supplementary Information
Supplementary data legends, methods, refs. 1–7 and Tables 1–6.
Supplementary Video 1
18F-FDG-PET/CT of SWNT-SHP1i treated and SWNT-Cy5.5 control apoE−/−mice.
Rights and permissions
About this article
Cite this article
Flores, A.M., Hosseini-Nassab, N., Jarr, KU. et al. Pro-efferocytic nanoparticles are specifically taken up by lesional macrophages and prevent atherosclerosis. Nat. Nanotechnol. 15, 154–161 (2020). https://doi.org/10.1038/s41565-019-0619-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-019-0619-3
This article is cited by
-
Emerging applications of single-cell profiling in precision medicine of atherosclerosis
Journal of Translational Medicine (2024)
-
Dysregulated cellular metabolism in atherosclerosis: mediators and therapeutic opportunities
Nature Metabolism (2024)
-
Selenopeptide nanomedicine ameliorates atherosclerosis by reducing monocyte adhesions and inflammations
Nano Research (2024)
-
Macrophage profiling in atherosclerosis: understanding the unstable plaque
Basic Research in Cardiology (2024)
-
Top Five Stories of the Cellular Landscape and Therapies of Atherosclerosis: Current Knowledge and Future Perspectives
Current Medical Science (2024)