Abstract
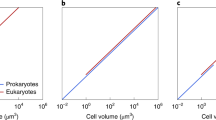
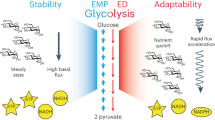
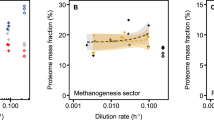
Mitochondria and aerobic respiration have been suggested to be required for the evolution of eukaryotic cell complexity. Aerobic respiration is several times more energetically efficient than fermentation. Moreover, aerobic respiration occurs at internalized mitochondrial membranes that are not constrained by a sublinear scaling with cell volume. However, diverse and complex anaerobic eukaryotes (for example, free-living and parasitic unicellular, and even small multicellular, eukaryotes) that exclusively rely on fermentation for energy generation have evolved repeatedly from aerobic ancestors. How do fermenting eukaryotes maintain their cell volumes and complexity while relying on such a low energy-yielding process? Here I propose that reduced rates of ATP generation in fermenting versus respiring eukaryotes are compensated for by longer cell cycles that satisfy lifetime energy demands. A literature survey and growth efficiency calculations show that fermenting eukaryotes divide approximately four to six times slower than aerobically respiring counterparts with similar cell volumes. Although ecological advantages such as competition avoidance offset lower growth rates and yields in the short term, fermenting eukaryotes inevitably have fewer physiological and ecological possibilities, which ultimately constrain their long-term evolutionary trajectories.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Margulis, L. Origin of Eukaryotic Cells (Yale Univ. Press, 1970).
Lane, N. & Martin, W. The energetics of genome complexity. Nature 467, 929–934 (2010).
Lane, N. How energy flow shapes cell evolution. Curr. Biol. 30, R471–R476 (2020).
Fenchel, T. & Finlay, B. J. The biology of free-living anaerobic ciliates. Eur. J. Protistol. 26, 201–215 (1991).
Müller, M. et al. Biochemistry and evolution of anaerobic energy metabolism in eukaryotes. Microbiol. Mol. Biol. Rev. 76, 444–495 (2012).
Stairs, C. W., Leger, M. M. & Roger, A. J. Diversity and origins of anaerobic metabolism in mitochondria and related organelles. Phil. Trans. R. Soc. B 370, 20140326 (2015).
Hampl, V., Čepička, I. & Eliáš, M. Was the mitochondrion necessary to start eukaryogenesis? Trends Microbiol. 27, 96–104 (2019).
Karnkowska, A. et al. A eukaryote without a mitochondrial organelle. Curr. Biol. 26, 1274–1284 (2016).
Fenchel, T. & Finlay, B. J. Ecology and Evolution in Anoxic Worlds (Oxford Univ. Press, 1995).
Stairs, C. W. et al. Microbial eukaryotes have adapted to hypoxia by horizontal acquisitions of a gene involved in rhodoquinone biosynthesis. eLife 7, e34292 (2018).
Woehle, C. et al. A novel eukaryotic denitrification pathway in foraminifera. Curr. Biol. 28, 2536–2543.e5 (2018).
Kamp, A., Høgslund, S., Risgaard-Petersen, N. & Stief, P. Nitrate storage and dissimilatory nitrate reduction by eukaryotic microbes. Front. Microbiol. 6, 1492 (2015).
Finlay, B. J., Span, A. S. W. & Harman, J. M. P. Nitrate respiration in primitive eukaryotes. Nature 303, 333–336 (1983).
Pánek, T. et al. Combined culture-based and culture-independent approaches provide insights into diversity of jakobids, an extremely plesiomorphic eukaryotic lineage. Front. Microbiol. 6, 1288 (2015).
Wylezich, C., Karpov, S. A., Mylnikov, A. P., Anderson, R. & Jürgens, K. Ecologically relevant choanoflagellates collected from hypoxic water masses of the Baltic Sea have untypical mitochondrial cristae. BMC Microbiol. 12, 271 (2012).
Yahalomi, D. et al. A cnidarian parasite of salmon (Myxozoa: Henneguya) lacks a mitochondrial genome. Proc. Natl Acad. Sci. USA 117, 5358–5363 (2020).
Stairs, C. W. et al. Anaeramoebae are a divergent lineage of eukaryotes that shed light on the transition from anaerobic mitochondria to hydrogenosomes. Curr. Biol. 31, 5605–5612.e5 (2021).
Rotterová, J. et al. Genomics of new ciliate lineages provides insight into the evolution of obligate anaerobiosis. Curr. Biol. 30, 2037–2050.e6 (2020).
Lane, N. & Martin, W. F. Eukaryotes really are special, and mitochondria are why. Proc. Natl Acad. Sci. USA 112, E4823 (2015).
Lane, N. Serial endosymbiosis or singular event at the origin of eukaryotes? J. Theor. Biol. 434, 58–67 (2017).
Booth, A. & Doolittle, W. F. Eukaryogenesis, how special really? Proc. Natl Acad. Sci. USA 112, 10278–10285 (2015).
Schavemaker, P. E. & Lynch, M. Flagellar energy costs across the tree of life. eLife 11, e77266 (2022).
Lynch, M., Schavemaker, P. E., Licknack, T. J., Hao, Y. & Pezzano, A. Evolutionary bioenergetics of ciliates. J. Eukaryot. Microbiol. https://doi.org/10.1111/jeu.12934 (2022).
Schavemaker, P. E. & Muñoz-Gómez, S. A. The role of mitochondrial energetics in the origin and diversification of eukaryotes. Nat. Ecol. Evol. 6, 1307–1317 (2022).
Lynch, M. & Marinov, G. K. Membranes, energetics, and evolution across the prokaryote-eukaryote divide. eLife 6, e20437 (2017).
Lynch, M. & Marinov, G. K. The bioenergetic costs of a gene. Proc. Natl Acad. Sci. USA 112, 15690–15695 (2015).
Fenchel, T. Respiration in heterotrophic unicellular eukaryotic organisms. Protist 165, 485–492 (2014).
Fenchel, T. & Finlay, B. J. Respiration rates in heterotrophic, free-living protozoa. Microb. Ecol. 9, 99–122 (1983).
Kiørboe, T. & Hirst, A. G. Shifts in mass scaling of respiration, feeding, and growth rates across life-form transitions in marine pelagic organisms. Am. Nat. 183, E118–E130 (2014).
Hatton, I. A., Dobson, A. P., Storch, D., Galbraith, E. D. & Loreau, M. Linking scaling laws across eukaryotes. Proc. Natl Acad. Sci. USA 116, 21616–21622 (2019).
Basan, M. et al. Overflow metabolism in Escherichia coli results from efficient proteome allocation. Nature 528, 99–104 (2015).
Chen, Y. & Nielsen, J. Energy metabolism controls phenotypes by protein efficiency and allocation. Proc. Natl Acad. Sci. USA 116, 17592–17597 (2019).
Malina, C., Yu, R., Björkeroth, J., Kerkhoven, E. J. & Nielsen, J. Adaptations in metabolism and protein translation give rise to the Crabtree effect in yeast. Proc. Natl Acad. Sci. USA 118, e2112836118 (2021).
Aguilera, A. & Benítez, T. Relationship between growth, fermentation, and respiration rates in Saccharomyces cerevisiae: a study based on the analysis of the yield Y(px). Biotechnol. Bioeng. 32, 240–244 (1988).
Fenchel, T. & Finlay, B. J. Anaerobic free-living protozoa: growth efficiencies and the structure of anaerobic communities. FEMS Microbiol. Lett. 74, 269–275 (1990).
Bauchop, T. & Elsden, S. R. The growth of micro-organisms in relation to their energy supply. Microbiology 23, 457–469 (1960).
Hirakata, Y. et al. Food selectivity of anaerobic protists and direct evidence for methane production using carbon from prey bacteria by endosymbiotic methanogen. ISME J. 14, 1873–1885 (2020).
Bernard, C. & Fenchel, T. Some microaerobic ciliates are facultative anaerobes. Eur. J. Protistol. 32, 293–297 (1996).
Nicholls, P. & Kim, J. K. Sulphide as an inhibitor and electron donor for the cytochrome c oxidase system. Can. J. Biochem. 60, 613–623 (1982).
Lele, U. N. & Watve, M. Bacterial growth rate and growth yield: is there a relationship? Proc. Indian Natl Sci. Acad. 30, 537–546 (2014).
Lipson, D. A. The complex relationship between microbial growth rate and yield and its implications for ecosystem processes. Front. Microbiol. 6, 615 (2015).
Pirt, S. J. The maintenance energy of bacteria in growing cultures. Proc. R. Soc. Lond. B 163, 224–231 (1965).
Fenchel, T. & Finlay, B. J. Endosymbiotic methanogenic bacteria in anaerobic ciliates: significance for the growth efficiency of the host. J. Protozool. 38, 18–22 (1991).
Muñoz-Gómez, S. A., Bilolikar, G., Wideman, J. G. & Geiler-Samerotte, K. Constructive neutral evolution 20 years later. J. Mol. Evol. 89, 172–182 (2021).
Archibald, J. M. Endosymbiosis and eukaryotic cell evolution. Curr. Biol. 25, R911–R921 (2015).
Baker, B. J. et al. Diversity, ecology and evolution of Archaea. Nat. Microbiol 5, 887–900 (2020).
Imachi, H. et al. Isolation of an archaeon at the prokaryote–eukaryote interface. Nature 577, 519–525 (2020).
Zaremba-Niedzwiedzka, K. et al. Asgard archaea illuminate the origin of eukaryotic cellular complexity. Nature 541, 353–358 (2017).
Shiratori, T., Suzuki, S., Kakizawa, Y. & Ishida, K. Phagocytosis-like cell engulfment by a planctomycete bacterium. Nat. Commun. 10, 5529 (2019).
Spang, A. et al. Proposal of the reverse flow model for the origin of the eukaryotic cell based on comparative analyses of Asgard archaeal metabolism. Nat. Microbiol. 4, 1138–1148 (2019).
Liu, Y. et al. Expanded diversity of Asgard archaea and their relationships with eukaryotes. Nature 593, 553–557 (2021).
Acknowledgements
I thank P. Schavemaker and M. Lynch (Arizona State University), and M. Leger (Institute of Evolutionary Biology, Barcelona) for providing comments on this manuscript. I apologize to those whose important contributions to the diversity of anaerobic eukaryotes have not been cited due to space constraints. This project was supported by an EMBO Postdoctoral Fellowship (ALTF 21-2020).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Peer review
Peer review information
Nature Microbiology thanks Courtney Stairs and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Tables 1 and 2
Table 1 Anaerobes (this study). Table 2 Aerobes (Fenchel, 1983).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Muñoz-Gómez, S.A. Energetics and evolution of anaerobic microbial eukaryotes. Nat Microbiol 8, 197–203 (2023). https://doi.org/10.1038/s41564-022-01299-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-022-01299-2