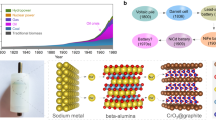
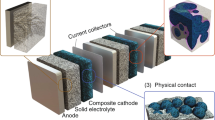
Abstract
Recent worldwide efforts to establish solid-state batteries as a potentially safe and stable high-energy and high-rate electrochemical storage technology still face issues with long-term performance, specific power and economic viability. Here, we review key challenges that still involve the need for fast-conducting solid electrolytes to provide sufficient transport in composite cathodes. In addition, we show that high-performance anodes together with protection concepts are paramount to establish dense high-energy solid-state batteries and that lithium-based solid-state batteries as well as metal anodes may not be the ultimate solution. We further discuss that diversity in terms of materials, research teams and approaches is key to establish long-term solid-state batteries. About ten years after the first ground-breaking publication of lithium solid electrolytes with an ionic conductivity higher than that of liquid electrolytes, it is time to realistically address the remaining key challenges for full-scale commercialization, cell performance and implementation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Betz, J. et al. Theoretical versus practical energy: a plea for more transparency in the energy calculation of different rechargeable battery systems. Adv. Energy Mater. 9, 1803170 (2019).
Solid-State Battery Roadmap 2035+ (Fraunhofer ISI, 2022).
Randau, S. et al. Benchmarking the performance of all-solid-state lithium batteries. Nat. Energy 5, 259–270 (2020). A first benchmarking study that suggests quantitative research targets for solid-state battery development.
Janek, J. & Zeier, W. G. A solid future for battery development. Nat. Energy 1, 16141 (2016).
Kerman, K., Luntz, A., Viswanathan, V., Chiang, Y.-M. & Chen, Z. Review—practical challenges hindering the development of solid state Li ion batteries. J. Electrochem. Soc. 164, A1731–A1744 (2017).
Pasta, M. et al. Energy 2020 roadmap on solid-state batteries. J. Phys. Energy 2, 32008 (2020).
Koerver, R. et al. Chemo-mechanical expansion of lithium electrode materials—on the route to mechanically optimized all-solid-state batteries. Energy Environ. Sci. 11, 2142–2158 (2018).
Ihrig, M. et al. Study of LiCoO2/Li7La3Zr2O12:Ta interface degradation in all-solid-state lithium batteries. ACS Appl. Mater. Int. 14, 11288–11299 (2022).
Ren, Y. et al. Oxide-based solid-state batteries: a perspective on composite cathode architecture. Adv. Energy Mater. https://doi.org/10.1002/aenm.202201939 (2023).
Krauskopf, T., Richter, F. H., Zeier, W. G. & Janek, J. Physico-chemical concepts of the lithium metal anode in solid-state batteries. Chem. Rev. 120, 7745–7794 (2020). A review on the properties and challenges of the lithium-metal anode in solid-state batteries.
Gao, X. et al. Solid-state lithium battery cathodes operating at low pressures. Joule 6, 636–646 (2022). A study highlighting the need and possibility to operate solid-state composites at low pressures.
Ohno, S., Rosenbach, C., Dewald, G. F., Janek, J. & Zeier, W. G. Linking solid electrolyte degradation to charge carrier transport in the thiophosphate-based composite cathode toward solid-state lithium–sulfur batteries. Adv. Funct. Mater. 31, 2010620 (2021).
Minnmann, P., Quillman, L., Burkhardt, S., Richter, F. H. & Janek, J. Quantifying the impact of charge transport bottlenecks in composite cathodes of all-solid-state batteries. J. Electrochem. Soc. 168, 040537 (2021). This work highlights the challenges of ionic and electronic charge transport in composite electrodes and provides guidelines to characterize these.
Bielefeld, A., Weber, D. A. & Janek, J. Modeling effective ionic conductivity and binder influence in composite cathodes for all-solid-state batteries. ACS Appl. Mater. Interfaces 12, 12821–12833 (2020).
Lee, Y. G. et al. High-energy long-cycling all-solid-state lithium metal batteries enabled by silver–carbon composite anodes. Nat. Energy 5, 299–308 (2020). Seminal study showing that zero lithium-excess solid-state batteries are possible with high energy densities.
Cronau, M., Duchardt, M., Szabo, M. & Roling, B. Ionic conductivity versus particle size of ball-milled sulfide-based solid electrolytes: strategy towards optimized composite cathode performance in all-solid-state batteries. Batter. Supercaps 5, e202200041 (2022).
Bielefeld, A., Weber, D. A., Rueß, R., Glavas, V. & Janek, J. Influence of lithium ion kinetics, particle morphology and voids on the electrochemical performance of composite cathodes for all-solid-state batteries. J. Electrochem. Soc. 169, 20539 (2022).
Minnmann, P. et al. Designing cathodes and cathode active materials for solid-state batteries. Adv. Energy Mater. 12, 2201425 (2022).
Nguyen, T. T. et al. The electrode tortuosity factor: why the conventional tortuosity factor is not well suited for quantifying transport in porous Li-ion battery electrodes and what to use instead. npj Comput. Mater. 6, 123 (2020).
Imholt, L. et al. Supramolecular self-assembly of methylated rotaxanes for solid polymer electrolyte application. ACS Macro Lett. 7, 881–885 (2018).
Dahbi, M., Ghamouss, F., Tran-Van, F., Lemordant, D. & Anouti, M. Comparative study of EC/DMC LiTFSI and LiPF6 electrolytes for electrochemical storage. J. Power Sources 196, 9743–9750 (2011).
Kato, Y. et al. All-solid-state batteries with thick electrode configurations. J. Phys. Chem. Lett. 9, 607–613 (2018). First study to highlight the need for faster solid electrolytes with conductivities over 10 mS cm–1 when high electrode loading is desired.
Froboese, L., Sichel, J. F., Van Der, L. T. & Helmers, L. Effect of microstructure on the ionic conductivity of an all solid-state battery electrode. J. Electrochem. Soc. 166, 318–328 (2019).
Bielefeld, A., Weber, D. A. & Janek, J. Microstructural modeling of composite cathodes for all-solid-state batteries. J. Phys. Chem. C 123, 1626–1634 (2019).
Kraft, M. A. et al. Inducing high ionic conductivity in the lithium superionic argyrodites Li6+xP1–xGexS5I for all-solid-state batteries. J. Am. Chem. Soc. 140, 16330–16339 (2018).
Zhou, L., Assoud, A., Zhang, Q., Wu, X. & Nazar, L. F. New family of argyrodite thioantimonate lithium superionic conductors. J. Am. Chem. Soc. 141, 19002–19013 (2019).
Kato, Y. et al. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 1, 16030 (2016).
Kamaya, N. et al. A lithium superionic conductor. Nat. Mater. 10, 682–686 (2011).
Riegger, L. M., Schlem, R., Sann, J., Zeier, W. G. & Janek, J. Lithium-metal anode instability of the superionic halide solid electrolytes and the implications for solid-state batteries. Angew. Chem. Int. Ed. 60, 6718–6723 (2021).
Wang, C. et al. A universal wet-chemistry synthesis of solid-state halide electrolytes for all-solid-state lithium-metal batteries. Sci. Adv. 7, eabh1896 (2022).
Nair, J. R., Imholt, L., Brunklaus, G. & Winter, M. Lithium metal polymer electrolyte batteries: opportunities and challenges. Electrochem. Soc. Interface 28, 55–61 (2019).
Weiss, M. et al. Fast charging of lithium-ion batteries: a review of materials aspects. Adv. Energy Mater. 11, 2101126 (2021).
Lewis, J. A., Cavallaro, K. A., Liu, Y. & McDowell, M. T. The promise of alloy anodes for solid-state batteries. Joule 6, 1418–1430 (2022).
Tan, D. H. S. et al. Carbon-free high-loading silicon anodes enabled by sulfide solid electrolytes. Science 373, 1494–1499 (2021). Seminal study showing the usage of Si metal anodes to be potentially competitive to Li metal.
Krauskopf, T. et al. The fast charge transfer kinetics of the lithium metal anode on the garnet-type solid electrolyte Li6.25Al0.25La3Zr2O12. Adv. Energy Mater. 10, 2000945 (2020).
Liu, X. et al. Local electronic structure variation resulting in Li ‘filament’ formation within solid electrolytes. Nat. Mater. 20, 1485–1490 (2021).
Krauskopf, T. et al. Lithium-metal growth kinetics on LLZO garnet-type solid electrolytes. Joule 3, 2030–2049 (2019).
Ning, Z. et al. Visualizing plating-induced cracking in lithium-anode solid-electrolyte cells. Nat. Mater. 20, 1121–1129 (2021).
Wang, M. J., Choudhury, R. & Sakamoto, J. Characterizing the Li-solid-electrolyte interface dynamics as a function of stack pressure and current density. Joule 3, 2165–2178 (2019).
Otto, S. K. et al. In situ investigation of lithium metal–solid electrolyte anode interfaces with ToF-SIMS. Adv. Mater. Interfaces 9, 2102387 (2022).
Lewis, J. A. et al. Role of areal capacity in determining short circuiting of sulfide-based solid-state batteries. ACS Appl. Mater. Int. 14, 4051–4060 (2022). Careful work highlighting the intrinsic challenges of using critical current densities as a metric.
Fuchs, T. et al. Increasing the pressure-free stripping capacity of the lithium metal anode in solid-state-batteries by carbon nanotubes. Adv. Energy Mater. 12, 2201125 (2022).
Kravchyk, K. V., Zhang, H., Okur, F. & Kovalenko, M. V. Li–garnet solid-state batteries with LLZO scaffolds. Acc. Mater. Res 3, 411–415 (2022).
Bloi, L. M. et al. Mechanistic insights into the reversible lithium storage in an open porous carbon via metal cluster formation in all solid-state batteries. Carbon 188, 325–335 (2022).
Schwietert, T. K. et al. Clarifying the relationship between redox activity and electrochemical stability in solid electrolytes. Nat. Mater. 19, 428–435 (2020).
Auvergniot, J. et al. Interface stability of argyrodite Li6PS5Cl toward LiCoO2, LiNi1/3Co1/3Mn1/3O2, and LiMn2O4 in bulk all-solid-state batteries. Chem. Mater. 29, 3883–3890 (2017).
Walther, F. et al. Visualization of the interfacial decomposition of composite cathodes in argyrodite-based all-solid-state batteries using time-of-flight secondary-ion mass spectrometry. Chem. Mater. 31, 3745–3755 (2019).
Dewald, G. F. et al. Experimental assessment of the practical oxidative stability of lithium thiophosphate solid electrolytes. Chem. Mater. 31, 8328–8337 (2019).
Culver, S. P., Koerver, R., Zeier, W. G. & Janek, J. On the functionality of coatings for cathode active materials in thiophosphate-based solid-state batteries. Adv. Energy Mater. 9, 1900626 (2019).
Sun, N. et al. Surface-to-bulk synergistic modification of single crystal cathode enables stable cycling of sulfide-based all-solid-state batteries at 4.4 V. Adv. Energy Mater. 12, 2200682 (2022). This work shows the need for changing active material particle sizes for their use in solid-state batteries.
Sendek, A. D. et al. Combining superionic conduction and favorable decomposition Products in the crystalline lithium−boron–sulfur system: a new mechanism for stabilizing solid Li-ion electrolytes. ACS Appl. Mater. Int. 12, 37957–37966 (2020).
Zhao, C. et al. Solid-state sodium batteries. Adv. Energy Mater. 8, 1703012 (2018).
Hayashi, A. et al. A sodium-ion sulfide solid electrolyte with unprecedented conductivity at room temperature. Nat. Commun. 10, 5266 (2019).
Fuchs, T., Culver, S. P., Till, P. & Zeier, W. G. Defect-mediated conductivity enhancements in Na3–xPn1–xWxS4 (Pn = P, Sb) using aliovalent substitutions. ACS Energy Lett. 5, 146–151 (2020).
Wenzel, S. et al. Interfacial reactivity benchmarking of the sodium ion conductors Na3PS4 and sodium β-alumina for protected sodium metal anodes and sodium all-solid-state batteries. ACS Appl. Mater. Int. 8, 28216–28224 (2016).
Zeier, W. G., Schlem, R., Banik, A., Eckardt, M. & Zobel, M. Na3–xEr1–xZrxCl6-A halide-based fast sodium-ion conductor with vacancy-driven ionic transport. ACS Appl. Energy Mater. 3, 10164–10173 (2020).
Wu, E. A. et al. A stable cathode-solid electrolyte composite for high-voltage, long-cycle-life solid-state sodium-ion batteries. Nat. Commun. 12, 1256 (2021).
Duchêne, L. et al. A highly stable sodium solid-state electrolyte based on a dodeca/deca-borate equimolar mixture. Chem. Commun. 53, 4195–4198 (2017).
Duchêne, L. et al. A stable 3 V all-solid-state sodium-ion battery based on a closo-borate electrolyte. Energy Environ. Sci. 10, 2609–2615 (2017).
Duchêne, L., Remhof, A., Hagemann, H. & Battaglia, C. Status and prospects of hydroborate electrolytes for all-solid-state batteries. Energy Storage Mater. 25, 782–794 (2020).
Lee, H. J. et al. LiNi0.5Mn1.5O4 cathode microstructure for all-solid-state batteries. Nano Lett. 22, 7477–7483 (2022).
Ohno, S. & Zeier, W. G. Toward practical solid-state lithium–sulfur batteries: challenges and perspectives. Acc. Mater. Res 2, 869–880 (2021). This perspective highlights the promising advances in Li–S-based solid-state batteries.
Santhosha, A. L. et al. Macroscopic displacement reaction of copper sulfide in lithium solid-state batteries. Adv. Energy Mater. 10, 2002394 (2020).
Dewald, G. F., Liaqat, Z., Lange, M. A., Tremel, W. & Zeier, W. G. Influence of iron sulfide nanoparticle sizes in solid-state batteries. Angew. Chem. Int. Ed. 60, 17952–17956 (2021).
Xu, S. et al. A high capacity all solid‐state Li–sulfur battery enabled by conversion‐intercalation hybrid cathode architecture. Adv. Funct. Mater. 31, 2004239 (2021).
Bettenhausen, C. Solid Power wins $12.5 million for FeS2 cathodes Chem. Eng. News (1 October 2021).
Tanibata, N., Deguchi, M., Hayashi, A. & Tatsumisago, M. All-solid-state Na/S batteries with a Na3PS4 electrolyte operating at room temperature. Chem. Mater. 29, 5232–5238 (2017).
Wang, C. et al. Boosting the performance of lithium batteries with solid–liquid hybrid electrolytes: interfacial properties and effects of liquid electrolytes. Nano Energy 48, 35–43 (2018).
Simon, F. J., Hanauer, M., Richter, F. H. & Janek, J. Interphase formation of PEO20:LiTFSI-Li6PS5Cl composite electrolytes with lithium metal. ACS Appl. Mater. Int. 12, 11713–11723 (2020).
Li, Y. et al. Thin solid electrolyte layers enabled by nanoscopic polymer binding. ACS Energy Lett. 5, 955–961 (2020).
Fuchs, T. et al. Working principle of an ionic liquid interlayer during pressureless lithium stripping on Li6.25Al0.25La3Zr2O12 (LLZO) garnet-type solid electrolyte. Batter. Supercaps 4, 1145–1155 (2021).
Schnell, J. et al. All-solid-state lithium-ion and lithium metal batteries—paving the way to large-scale production. J. Power Sources 382, 160–175 (2018).
Miura, A. et al. Liquid-phase syntheses of sulfide electrolytes for all-solid-state lithium battery. Nat. Rev. Chem. 3, 189–198 (2019).
Ghidiu, M., Ruhl, J., Culver, S. P. & Zeier, W. G. Solution-based synthesis of lithium thiophosphate superionic conductors for solid-state batteries: a chemistry perspective. J. Mater. Chem. A 7, 17735–17753 (2019).
Li, X. et al. Air-stable Li3InCl6 electrolyte with high voltage compatibility for all-solid-state batteries. Energy Environ. Sci. 12, 2665–2671 (2019).
Rosenbach, C. et al. Visualizing the chemical incompatibility of halide and sulfide-based electrolytes in solid-state batteries. Adv. Energy Mater. https://doi.org/10.1002/aenm.202203673 (2022).
Jung, K. N., Shin, H. S., Park, M. S. & Lee, J. W. Solid-state lithium batteries: bipolar design, fabrication, and electrochemistry. ChemElectroChem 6, 3842–3859 (2019).
Azhari, L., Bong, S., Ma, X. & Wang, Y. Recycling for all solid-state lithium-ion. Batter. Matter 3, 1845–1861 (2020).
Duffner, F. et al. Post-lithium-ion battery cell production and its compatibility with lithium-ion cell production infrastructure. Nat. Energy 6, 123–134 (2021).
Bates, A. M. et al. Are solid-state batteries safer than lithium-ion batteries? Joule 6, 742–755 (2022). First study to approach the question of device safety concerns in solid-state batteries.
Kim, T. et al. Thermal runaway behavior of Li6PS5Cl solid electrolytes for LiNi0.8Co0.1Mn0.1O2 and LiFePO4 in all-solid-state batteries. Chem. Mater. 34, 9159–9171 (2022).
Ohno, S. et al. How certain are the reported ionic conductivities of thiophosphate-based solid electrolytes? An interlaboratory study. ACS Energy Lett. 5, 910–915 (2020).
Kato, Y. et al. Synthesis, structure and lithium ionic conductivity of solid solutions of Li10(Ge1–xMx)P2S12 (M = Si, Sn). J. Power Sources 271, 60–64 (2014).
Hori, S. et al. Synthesis, structure, and ionic conductivity of solid solution, Li10+δM1+δP2–δS12 (M = Si, Sn). Faraday Discuss. 176, 83–94 (2014).
Sun, Y., Suzuki, K., Hori, S., Hirayama, M. & Kanno, R. Superionic conductors: Li10+δ[SnySi1–y]1+δP2–δS12 with a Li10GeP2S12-type structure in the Li3PS4–Li4SnS4–Li4SiS4 quasi-ternary system. Chem. Mater. 29, 5858–5864 (2017).
Sun, Y. et al. Oxygen substitution effects in Li10GeP2S12 solid electrolyte. J. Power Sources 324, 798–803 (2016).
Kwon, O. et al. Synthesis, structure, and conduction mechanism of the lithium superionic conductor Li10+δGe1+δP2–δS12. J. Mater. Chem. A 3, 438–446 (2015).
Krauskopf, T., Culver, S. P. & Zeier, W. G. Bottleneck of diffusion and inductive effects in Li10Ge1– xSnxP2S12. Chem. Mater. 30, 1791–1798 (2018).
Bron, P. et al. Li10SnP2S12—an affordable lithium superionic conductor. J. Am. Chem. Soc. 135, 15694–15697 (2013).
Iwasaki, R. et al. Weak anisotropic lithium-ion conductivity in single crystals of Li10GeP2S12. Chem. Mater. 31, 3694–3699 (2019).
Suzuki, K. et al. Synthesis, structure, and electrochemical properties of crystalline Li–P–S–O solid electrolytes: novel lithium-conducting oxysulfides of Li10GeP2S12 family. Solid State Ion. 288, 229–234 (2016).
Hori, S., Suzuki, K., Hirayama, M., Kato, Y. & Kanno, R. Lithium superionic conductor Li9.42Si1.02P2.1S9.96O2.04 with Li10GeP2S12 -type structure in the Li2S–P2S5–SiO2 pseudoternary system: synthesis, electrochemical properties, and structure–composition relationships. Front. Energy Res. 4, 1–9 (2016).
Liang, J. et al. Li10Ge(P1–xSbx)2S12 lithium-ion conductors with enhanced atmospheric stability. Chem. Mater. 32, 2664–2672 (2020).
Hori, S. et al. Structure–property relationships in lithium superionic conductors having a Li10GeP2S12-type structure. Acta Crystallogr. B 71, 727–736 (2015).
Inagaki, M. et al. Conduction mechanism of Li10GeP2S12-type lithium superionic conductors in a Li–Sn–Si–P–S system. Chem. Mater. 31, 3485–3490 (2019).
Kuhn, A., Duppel, V. & Lotsch, B. V. Tetragonal Li10GeP2S12 and Li7GePS8-exploring the Li ion dynamics in LGPS Li electrolytes. Energy Environ. Sci. 6, 3548–3552 (2013).
Kuhn, A. et al. A new ultrafast superionic Li-conductor: ion dynamics in Li11Si2PS12 and comparison with other tetragonal LGPS-type electrolytes. Phys. Chem. Chem. Phys. 16, 14669–14674 (2014).
Kim, K. H. & Martin, S. W. Structures and properties of oxygen-substituted Li10SiP2S12–xOx solid-state electrolytes. Chem. Mater. 31, 3984–3991 (2019).
Kraft, M. A. et al. Influence of lattice polarizability on the ionic conductivity in the lithium superionic argyrodites Li6PS5X (X = Cl, Br, I). J. Am. Chem. Soc. 139, 10909–10918 (2017).
Sakuda, A. et al. Mechanochemically prepared Li2S–P2S5–LiBH4 solid electrolytes with an argyrodite structure. ACS Omega 3, 5453–5458 (2018).
Minafra, N., Culver, S. P., Krauskopf, T., Senyshyn, A. & Zeier, W. G. Effect of Si substitution on the structural and transport properties of superionic Li-argyrodites. J. Mater. Chem. A 6, 645–651 (2018).
Mizuno, F., Hayashi, A., Tadanaga, K. & Tatsumisago, M. High lithium ion conducting glass–ceramics in the system Li2S–P2S5. Solid State Ion. 177, 2721–2725 (2006).
Zeier, W. G., Zhou, S., Lopez-Bermudez, B., Page, K. & Melot, B. C. Dependence of the Li-ion conductivity and activation energies on the crystal structure and ionic radii in Li6MLa2Ta2O12. ACS Appl. Mater. Interfaces 6, 10900–10907 (2014).
Cussen, E. J., O’Callaghan, M. P., Powell, A. S., Titman, J. J. & Chen, G. Z. Switching on fast lithium ion conductivity in garnets: the structure and transport properties of Li3+xNd3Te2–xSbxO12. Chem. Mater. 20, 2360–xO2369 (2008).
Wu, J. F. et al. Gallium-doped Li7La3Zr2O12 garnet-type electrolytes with high lithium-ion conductivity. ACS Appl. Mater. Interfaces 9, 1542–1552 (2017).
Logéat, A. et al. From order to disorder: the structure of lithium-conducting garnets Li7–xLa3TaxZr2–xO12 (x = 0–2). Solid State Ion. 206, 33–38 (2012).
Ohta, S., Kobayashi, T. & Asaoka, T. High lithium ionic conductivity in the garnet-type oxide Li7–xLa3(Zr2–x,Nbx)O12 (x = 0–2). J. Power Sources 196, 3342–3345 (2011).
Rettenwander, D. et al. Synthesis, crystal chemistry, and electrochemical properties of Li7–2xLa3Zr2–xMoxO12 (x = 0.1–0.4): stabilization of the cubic garnet polymorph via substitution of Zr4+ by Mo6+. Inorg. Chem. 54, 10440–10449 (2015).
Li, Y., Han, J. T., Wang, C. A., Xie, H. & Goodenough, J. B. Optimizing Li+ conductivity in a garnet framework. J. Mater. Chem. 22, 15357–15361 (2012).
Awaka, J., Kijima, N., Hayakawa, H. & Akimoto, J. Synthesis and structure analysis of tetragonal Li7La3Zr2O12 with the garnet-related type structure. J. Solid State Chem. 182, 2046–2052 (2009).
Percival, J., Kendrick, E. & Slater, P. R. Synthesis and characterisation of the garnet-related Li ion conductor, Li5Nd3Sb2O12. Mater. Res. Bull. 43, 765–770 (2008).
Zhao, G., Suzuki, K., Yonemura, M., Hirayama, M. & Kanno, R. Enhancing fast lithium ion conduction in Li4GeO4–Li3PO4 solid electrolytes. ACS Appl. Energy Mater. 2, 6608–6615 (2019).
Deng, Y. et al. Enhancing the lithium ion conductivity in lithium superionic conductor (LISICON) solid electrolytes through a mixed polyanion effect. ACS Appl. Mater. Interfaces 9, 7050–7058 (2017).
Deng, Y. et al. Structural and mechanistic insights into fast lithium-ion conduction in Li4SiO4–Li3PO4 solid electrolytes. J. Am. Chem. Soc. 137, 9136–9145 (2015).
Hong, H. Y. P. Crystal structure and ionic conductivity of Li14Zn(GeO4)4 and other new Li+ superionic conductors. Mater. Res. Bull. 13, 117–124 (1978).
Pérez-Estébanez, M., Isasi-Marín, J., Többens, D. M., Rivera-Calzada, A. & León, C. A systematic study of NASICON-type Li1+xMxTi2–x(PO4)3 (M: Cr, Al, Fe) by neutron diffraction and impedance spectroscopy. Solid State Ion. 266, 1–8 (2014).
Weiss, M., Weber, D. A., Senyshyn, A., Janek, J. & Zeier, W. G. Correlating transport and structural properties in Li1+xAlxGe2−x(PO4)3 (LAGP) prepared from aqueous solution. ACS Appl. Mater. Int. 10, 10939–10944 (2018).
Schlem, R. et al. Mechanochemical synthesis: a tool to tune cation site disorder and ionic transport properties of Li3MCl6 (M = Y, Er) superionic conductors. Adv. Energy Mater. 10, 1903719 (2019).
Asano, T. et al. Solid halide electrolytes with high lithium-ion conductivity for application in 4 V class bulk-type all-solid-state batteries. Adv. Mater. 30, 1803075 (2018).
Park, K. H. et al. High-voltage superionic halide solid electrolytes for all-solid-state Li-ion batteries. ACS Energy Lett. 5, 533–539 (2020).
Imholt, L. et al. Grafted polyrotaxanes as highly conductive electrolytes for lithium metal batteries. J. Power Sources 409, 148–158 (2019).
Butzelaar, A. J. et al. The power of architecture-cage-shaped PEO and its application as a polymer electrolyte. Polym. Chem. 12, 4326–4331 (2021).
Wu, N. et al. Enhanced surface interactions enable fast Li+ conduction in oxide/polymer composite electrolyte. Angew. Chem. Int. Ed. 59, 4131–4137 (2020).
Wang, W., Yi, E., Fici, A. J., Laine, R. M. & Kieffer, J. Lithium ion conducting poly(ethylene oxide)-based solid electrolytes containing active or passive ceramic nanoparticles. J. Phys. Chem. C 121, 2563–2573 (2017).
Chung, S. H. et al. Enhancement of ion transport in polymer electrolytes by addition of nanoscale inorganic oxides. J. Power Sources 97–98, 644–648 (2001).
Ding, M. S. & Jow, T. R. Conductivity and viscosity of PC–DEC and PC–EC solutions of LiPF6. J. Electrochem. Soc. 150, A620 (2003).
Kirillov, S. A., Gorobets, M. I., Tretyakov, D. O., Ataev, M. B. & Gafurov, M. M. Phase diagrams and conductivity of lithium salt systems in dimethyl sulfoxide, propylene carbonate and dimethyl carbonate. J. Mol. Liq. 205, 78–84 (2015).
Nyman, A., Behm, M. & Lindbergh, G. Electrochemical characterisation and modelling of the mass transport phenomena in LiPF6–EC–EMC electrolyte. Electrochim. Acta 53, 6356–6365 (2008).
Acknowledgements
We acknowledge financial support within the cluster of competence FESTBATT funded by Bundesministerium für Bildung und Forschung (BMBF; projects 03XP0431, 03XP0430A and 03XP0430F). We thank P. Till for support in data analyses.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Marm Dixit, Taro Hitosugi, Jennifer Rupp and Fan Wu for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Source Data Fig. 3
Supplementary tables for Fig. 3.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Janek, J., Zeier, W.G. Challenges in speeding up solid-state battery development. Nat Energy 8, 230–240 (2023). https://doi.org/10.1038/s41560-023-01208-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-023-01208-9
This article is cited by
-
External-pressure–electrochemistry coupling in solid-state lithium metal batteries
Nature Reviews Materials (2024)
-
Metal electrodes for next-generation rechargeable batteries
Nature Reviews Electrical Engineering (2024)
-
Interfacial self-healing polymer electrolytes for long-cycle solid-state lithium-sulfur batteries
Nature Communications (2024)
-
Creep-type all-solid-state cathode achieving long life
Nature Communications (2024)
-
Recycling of solid-state batteries
Nature Energy (2024)