Abstract
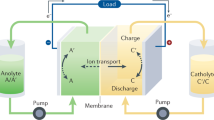
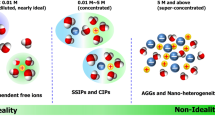
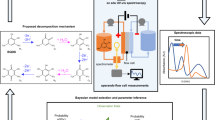
Redox flow batteries (RFBs) are a promising technology for large-scale energy storage. Rapid research developments in RFB chemistries, materials and devices have laid critical foundations for cost-effective and long-lasting RFB systems. However, the lack of consistency in testing methods and assessment metrics makes it challenging to compare reported RFBs and evaluate their potential for practical applications. Here we discuss RFB assessment methods and performance metrics in direct relation to their working principles and degradation mechanisms. We first introduce basic cell attributes and performance metrics and describe common misconceptions in testing and performance comparison. We discuss major RFB decay mechanisms and highlight bottlenecks in organic, inorganic and solid-hybrid RFBs. Testing protocols, reporting practices and comparison criteria are proposed under a general framework of symmetric and asymmetric full RFBs. These recommendations can be broadly applied to a wide range of flow battery chemistries to facilitate future benchmarking and RFB development.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Park, M., Ryu, J., Wang, W. & Cho, J. Material design and engineering of next-generation flow-battery technologies. Nat. Rev. Mater. 2, 16080 (2016).
Li, L. et al. A stable vanadium redox-flow battery with high energy density for large-scale energy storage. Adv. Energy Mater. 1, 394–400 (2011).
Janoschka, T. et al. An aqueous, polymer-based redox-flow battery using non-corrosive, safe, and low-cost materials. Nature 527, 78–81 (2015).
Huskinson, B. et al. A metal-free organic–inorganic aqueous flow battery. Nature 505, 195–198 (2014).
Li, Z., Weng, G., Zou, Q., Cong, G. & Lu, Y.-C. A high-energy and low-cost polysulfide/iodide redox flow battery. Nano Energy 30, 283–292 (2016).
Xie, C., Liu, Y., Lu, W., Zhang, H. & Li, X. Highly stable zinc-iodine single flow batteries with super high energy density for stationary energy storage. Energy Environ. Sci. 12, 1834–1839 (2019).
Li, B. et al. Ambipolar zinc-polyiodide electrolyte for a high-energy density aqueous redox flow battery. Nat. Commun. 6, 6303 (2015).
Weng, G.-M., Li, Z., Cong, G., Zhou, Y. & Lu, Y.-C. Unlocking the capacity of iodide for high-energy-density zinc/polyiodide and lithium/polyiodide redox flow batteries. Energy Environ. Sci. 10, 735–741 (2017).
Zhao, Y. et al. A chemistry and material perspective on lithium redox flow batteries towards high-density electrical energy storage. Chem. Soc. Rev. 44, 7968–7996 (2015).
Xie, C. et al. A highly reversible neutral zinc/manganese battery for stationary energy storage. Energy Environ. Sci. 13, 135–143 (2020).
Wang, Z., Tam, L.-Y. S. & Lu, Y.-C. Flexible solid flow electrodes for high-energy scalable energy storage. Joule 3, 1677–1688 (2019).
Kwabi, D. G., Ji, Y. & Aziz, M. J. Electrolyte lifetime in aqueous organic redox flow batteries: a critical review. Chem. Rev. 120, 6467–6489 (2020).
Xiao, J. et al. Understanding and applying Coulombic efficiency in lithium metal batteries. Nat. Energy 5, 561–568 (2020).
Darling, R., Gallagher, K., Xie, W., Su, L. & Brushett, F. Transport property requirements for flow battery separators. J. Electrochem. Soc. 163, A5029–A5040 (2015).
Chen, Q., Eisenach, L. & Aziz, M. J. Cycling analysis of a quinone-bromide redox flow battery. J. Electrochem. Soc. 163, A5057–A5063 (2016).
Tong, L. et al. UV-vis spectrophotometry of quinone flow battery electrolyte for in situ monitoring and improved electrochemical modeling of potential and quinhydrone formation. Phys. Chem. Chem. Phys. 19, 31684–31691 (2017).
Skyllas‐Kazacos, M., Kazacos, G., Poon, G. & Verseema, H. Recent advances with UNSW vanadium‐based redox flow batteries. Int. J. Energy Res. 34, 182–189 (2010).
Perry, M. L., Saraidaridis, J. D. & Darling, R. M. Crossover mitigation strategies for redox-flow batteries. Curr. Opin. Electrochem. 21, 311–318 (2020).
Waters, S. E., Robb, B. H. & Marshak, M. P. Effect of chelation on iron-chromium redox flow batteries. ACS Energy Lett. 5, 1758–1762 (2020).
Jin, S. et al. Near neutral pH redox flow battery with low permeability and long‐lifetime phosphonated viologen active species. Adv. Energy Mater. 10, 2000100 (2020).
Dong, Y.-R., Kaku, H., Hanafusa, K., Moriuchi, K. & Shigematsu, T. A novel titanium/manganese redox flow battery. ECS Trans. 69, 59–67 (2015).
Yuan, Z. et al. Advanced porous membranes with ultra-high selectivity and stability for vanadium flow batteries. Energy Environ. Sci. 9, 441–447 (2016).
Zhou, H., Zhang, H., Zhao, P. & Yi, B. A comparative study of carbon felt and activated carbon based electrodes for sodium polysulfide/bromine redox flow battery. Electrochim. Acta 51, 6304–6312 (2006).
Ma, D. et al. Highly active nanostructured CoS2/CoS heterojunction electrocatalysts for aqueous polysulfide/iodide redox flow batteries. Nat. Commun. 10, 3367 (2019).
Goulet, M.-A. & Aziz, M. J. Flow battery molecular reactant stability determined by symmetric cell cycling methods. J. Electrochem. Soc. 165, A1466–A1477 (2018).
Zhang, C. et al. Phenothiazine-based organic catholyte for high-capacity and long-life aqueous redox flow batteries. Adv. Mater. 31, 1901052 (2019).
Park, M. et al. A high voltage aqueous zinc–organic hybrid flow battery. Adv. Energy Mater. 9, 1900694 (2019).
Beh, E. S. et al. A neutral pH aqueous organic–organometallic redox flow battery with extremely high capacity retention. ACS Energy Lett. 2, 639–644 (2017).
Kwabi, D. G. et al. Alkaline quinone flow battery with long lifetime at pH 12. Joule 2, 1894–1906 (2018).
Lin, K. et al. A redox-flow battery with an alloxazine-based organic electrolyte. Nat. Energy 1, 16102 (2016).
Liu, T., Wei, X., Nie, Z., Sprenkle, V. & Wang, W. A total organic aqueous redox flow battery employing a low cost and sustainable methyl viologen anolyte and 4‐HO‐TEMPO catholyte. Adv. Energy Mater. 6, 1501449 (2016).
Liu, Y. et al. A long-lifetime all-organic aqueous flow battery utilizing TMAP-TEMPO radical. Chem 5, 1861–1870 (2019).
Hu, B., DeBruler, C., Rhodes, Z. & Liu, T. L. Long-cycling aqueous organic redox flow battery (AORFB) toward sustainable and safe energy storage. J. Am. Chem. Soc. 139, 1207–1214 (2017).
Yang, B. et al. High-performance aqueous organic flow battery with quinone-based redox couples at both electrodes. J. Electrochem. Soc. 163, A1442–A1449 (2016).
Goulet, M.-A. et al. Extending the lifetime of organic flow batteries via redox state management. J. Am. Chem. Soc. 141, 8014–8019 (2019).
Tong, L. et al. Molecular engineering of an alkaline naphthoquinone flow battery. ACS Energy Lett. 4, 1880–1887 (2019).
Xie, C., Duan, Y., Xu, W., Zhang, H. & Li, X. A low-cost neutral zinc-iron flow battery with high energy density for stationary energy storage. Angew. Chem. Int. Ed. 56, 14953–14957 (2017).
Roe, S., Menictas, C. & Skyllas-Kazacos, M. A high energy density vanadium redox flow battery with 3 M vanadium electrolyte. J. Electrochem. Soc. 163, A5023–A5028 (2015).
Hollas, A. et al. A biomimetic high-capacity phenazine-based anolyte for aqueous organic redox flow batteries. Nat. Energy 3, 508–514 (2018).
Li, Z. & Lu, Y.-C. Material design of aqueous redox flow batteries: fundamental challenges and mitigation strategies. Adv. Mater. 32, 2002132 (2020).
Lee, J.-H. et al. Dendrite-free Zn electrodeposition triggered by interatomic orbital hybridization of Zn and single vacancy carbon defects for aqueous Zn-based flow batteries. Energy Environ. Sci. 13, 2839–2848 (2020).
Yin, Y. et al. Dendrite-free zinc deposition induced by tin-modified multifunctional 3D host for stable zinc-based flow battery. Adv. Mater. 32, 1906803 (2020).
Darling, R. M. & Perry, M. L. Half-cell, steady-state flow-battery experiments. ECS Trans. 53, 31–38 (2013).
Ke, X. et al. Rechargeable redox flow batteries: flow fields, stacks and design considerations. Chem. Soc. Rev. 47, 8721–8743 (2018).
Zheng, Q., Xing, F., Li, X., Ning, G. & Zhang, H. Flow field design and optimization based on the mass transport polarization regulation in a flow-through type vanadium flow battery. J. Power Sources 324, 402–411 (2016).
Brushett, F. R., Aziz, M. J. & Rodby, K. E. On lifetime and cost of redox-active organics for aqueous flow batteries. ACS Energy Lett. 5, 879–884 (2020).
Jin, S. et al. A water-miscible quinone flow battery with high volumetric capacity and energy density. ACS Energy Lett. 4, 1342–1348 (2019).
Wang, C. et al. Molecular design of fused-ring phenazine derivatives for long-cycling alkaline redox flow batteries. ACS Energy Lett. 5, 411–417 (2020).
Wu, M. et al. Extremely stable anthraquinone negolytes synthesized from common precursors. Chem 6, 1432–1442 (2020).
Yuan, Z., Duan, Y., Liu, T., Zhang, H. & Li, X. Toward a low-cost alkaline zinc-iron flow battery with a polybenzimidazole custom membrane for stationary energy storage. iScience 3, 40–49 (2018).
Acknowledgements
The work described herein was supported by a grant from the Research Grant Council (RGC) of the Hong Kong Special Administrative Region, China (project number T23-601/17-R).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Energy thanks Travis Anderson, Mike Perry and Wei Wang for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Source Data Fig. 2
Numerical data used to generate the graphs in Fig. 2c,d.
Rights and permissions
About this article
Cite this article
Yao, Y., Lei, J., Shi, Y. et al. Assessment methods and performance metrics for redox flow batteries. Nat Energy 6, 582–588 (2021). https://doi.org/10.1038/s41560-020-00772-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-020-00772-8
This article is cited by
-
Starch-mediated colloidal chemistry for highly reversible zinc-based polyiodide redox flow batteries
Nature Communications (2024)
-
Mild pH-decoupling aqueous flow battery with practical pH recovery
Nature Energy (2024)
-
Accelerating discovery in organic redox flow batteries
Nature Computational Science (2024)
-
An active and durable molecular catalyst for aqueous polysulfide-based redox flow batteries
Nature Energy (2023)
-
A solution-to-solid conversion chemistry enables ultrafast-charging and long-lived molten salt aluminium batteries
Nature Communications (2023)