Abstract
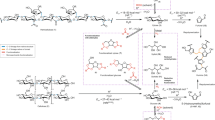
The development of sustainable plastics from abundant renewable feedstocks has been limited by the complexity and efficiency of their production, as well as their lack of competitive material properties. Here we demonstrate the direct transformation of the hemicellulosic fraction of non-edible biomass into a tricyclic diester plastic precursor at 83% yield (95% from commercial xylose) during integrated plant fractionation with glyoxylic acid. Melt polycondensation of the resulting diester with a range of aliphatic diols led to amorphous polyesters (Mn = 30–60 kDa) with high glass transition temperatures (72–100 °C), tough mechanical properties (ultimate tensile strengths of 63–77 MPa, tensile moduli of 2,000–2,500 MPa and elongations at break of 50–80%) and strong gas barriers (oxygen transmission rates (100 µm) of 11–24 cc m−2 day−1 bar−1 and water vapour transmission rates (100 µm) of 25–36 g m−2 day−1) that could be processed by injection moulding, thermoforming, twin-screw extrusion and three-dimensional printing. Although standardized biodegradation studies still need to be performed, the inherently degradable nature of these materials facilitated their chemical recycling via methanolysis at 64 °C, and eventual depolymerization in room-temperature water.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data needed to support the findings of this study are included in the main text or in the Supplementary Information. Crystallographic data for the structure reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition no. CCDC 2121378 (1S-DMGX). Copies of the data can be obtained free of charge via ccdc.cam.ac.uk/structures/Search?ccdc=2121378. All of the data associated with the Article have been deposited38 with Zenodo at https://zenodo.org/record/6482769.
References
Geyer, R., Jambeck, J. R. & Law, K. L. Production, use and fate of all plastics ever made. Sci. Adv. 3, e1700782 (2017).
Hillmyer, M. A. The promise of plastics from plants. Science 358, 868–870 (2017).
Zhu, Y., Romain, C. & Williams, C. K. Sustainable polymers from renewable resources. Nature 540, 354–362 (2016).
Tang, X. & Chen, E. Y.-X. Chemical synthesis of perfectly isotactic and high melting bacterial poly(3-hydroxybutyrate) from bio-sourced racemic cyclic diolide. Nat. Commun. 9, 2345 (2018).
Tang, X., Westlie, A. H., Watson, E. M. & Chen, E. Y.-X. Stereosequenced crystalline polyhydroxyalkanoates from diastereomeric monomer mixtures. Science 366, 754–758 (2019).
Wypych, G. Handbook of Polymers (Elsevier, 2016).
Rose, M. & Palkovits, R. Isosorbide as a renewable platform chemical for versatile applications—quo vadis? ChemSusChem 5, 167–176 (2012).
Muñoz-Guerra, S., Lavilla, C., Japu, C. & Martínez de Ilarduya, A. Renewable terephthalate polyesters from carbohydrate-based bicyclic monomers. Green Chem. 16, 1716–1739 (2014).
Stadler, B. M., Wulf, C., Werner, T., Tin, S. & de Vries, J. G. Catalytic approaches to monomers for polymers based on renewables. ACS Catal. 9, 8012–8067 (2019).
Sajid, M., Zhao, X. & Liu, D. Production of 2,5-furandicarboxylic acid (FDCA) from 5-hydroxymethylfurfural (HMF): recent progress focusing on the chemical-catalytic routes. Green Chem. 20, 5427–5453 (2018).
Shahzadi, T. et al. Advances in lignocellulosic biotechnology: a brief review on lignocellulosic biomass and cellulases. Adv. Biosci. Biotechnol. 05, 246–251 (2014).
Naik, S. N., Goud, V. V., Rout, P. K. & Dalai, A. K. Production of first and second generation biofuels: a comprehensive review. Renew. Sust. Energ. Rev. 14, 578–597 (2010).
Häußler, M., Eck, M., Rothauer, D. & Mecking, S. Closed-loop recycling of polyethylene-like materials. Nature 590, 423–427 (2021).
Upton, B. M. & Kasko, A. M. Strategies for the conversion of lignin to high-value polymeric materials: review and perspective. Chem. Rev. 116, 2275–2306 (2016).
Cywar, R. M., Rorrer, N. A., Hoyt, C. B., Beckham, G. T. & Chen, E. Y.-X. Bio-based polymers with performance-advantaged properties. Nat. Rev. Mater. 7, 83–103 (2022).
Shuai, L. et al. Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerization. Science 354, 329–333 (2016).
Lan, W., Amiri, M. T., Hunston, C. M. & Luterbacher, J. S. Protection group effects during α,γ-diol lignin stabilization promote high-selectivity monomer production. Angew. Chem. Int. Ed. 57, 1356–1360 (2018).
Questell-Santiago, Y. M., Zambrano-Varela, R., Talebi Amiri, M. & Luterbacher, J. S. Carbohydrate stabilization extends the kinetic limits of chemical polysaccharide depolymerization. Nat. Chem. 10, 1222–1228 (2018).
Talebi Amiri, M., Dick, G. R., Questell-Santiago, Y. M. & Luterbacher, J. S. Fractionation of lignocellulosic biomass to produce uncondensed aldehyde-stabilized lignin. Nat. Protoc. 14, 921–954 (2019).
Biddy, M. J., Scarlata, C. & Kinchin, C. Chemicals from Biomass: A Market Assessment of Bioproducts with Near-Term Potential. NREL/TP–5100-65509, 1244312 (OSTI, 2016); http://www.osti.gov/servlets/purl/1244312/; https://doi.org/10.2172/1244312
Pointner, M., Kuttner, P. & Obrlik, T. Composition of corncobs as a substrate for fermentation of biofuels. Agron. Res. 12, 391–396 (2014).
Cheng, M.-T. et al. Particulate matter characteristics during agricultural waste burning in Taichung City, Taiwan. J. Hazard. Mater. 165, 187–192 (2009).
Van Nieuwenhove, I. et al. Biobased resins using lignin and glyoxal. ACS Sustain. Chem. Eng. 8, 18789–18809 (2020).
Pickett, D. J. & Yap, K. S. A study of the production of glyoxylic acid by the electrochemical reduction of oxalic acid solutions. J. Appl. Electrochem. 4, 17–23 (1974).
Scharbert, B. & Babusiaux, P. Electrochemical process for reducing oxalic acid to glyoxylic acid. US patent US005395488A (1995).
Schuler, E., Demetriou, M., Shiju, N. R. & Gruter, G.-J. M. Towards sustainable oxalic acid from CO2 and biomass. ChemSusChem 14, 3636–3664 (2021).
Davis, R. et al. Process Design and Economics for the Conversion of Lignocellulosic Biomass to Hydrocarbons: Dilute-Acid and Enzymatic Deconstruction of Biomass to Sugars and Catalytic Conversion of Sugars to Hydrocarbons. NREL/TP–5100-62498, 1176746 (OSTI, 2015); http://www.osti.gov/servlets/purl/1176746/; https://doi.org/10.2172/1176746
Motagamwala, A. H. et al. Toward biomass-derived renewable plastics: production of 2,5-furandicarboxylic acid from fructose. Sci. Adv. 4, eaap9722 (2018).
Straathof, A. J. J. & Bampouli, A. Potential of commodity chemicals to become bio-based according to maximum yields and petrochemical prices. Biofuels Bioprod. Biorefining 11, 798–810 (2017).
Lange, J. & Wyser, Y. Recent innovations in barrier technologies for plastic packaging—a review. Packag. Technol. Sci. 16, 149–158 (2003).
George, N. & Kurian, T. Recent developments in the chemical recycling of postconsumer poly(ethylene terephthalate) waste. Ind. Eng. Chem. Res. 53, 14185–14198 (2014).
Stloukal, P., Jandikova, G., Koutny, M. & Sedlařík, V. Carbodiimide additive to control hydrolytic stability and biodegradability of PLA. Polym. Test. 54, 19–28 (2016).
Sato, H., Akutsu, F., Kobayashi, F. & Okada, Y. Process for producing aliphatic polyester. US patent US7538178B2 (2009).
Glyoxylic acid—Registration Dossier [online] (ECHA, accessed 29 March 2021); https://echa.europa.eu/registration-dossier/-/registered-dossier/14954/6/2/8
1,4-Butanediol—Registration Dossier [online] (ECHA, accessed 29 March 2021); https://echa.europa.eu/registration-dossier/-/registered-dossier/15496/1
1,5-Pentanediol—Registration Dossier [online] (ECHA, accessed 29 March 2021); https://echa.europa.eu/registration-dossier/-/registered-dossier/14818/6/2/8
1,6-Hexanediol—Registration Dossier [online] (ECHA, accessed 29 March 2021); https://echa.europa.eu/registration-dossier/-/registered-dossier/15109/6/2/1
Acknowledgements
This work was supported by the Swiss National Science Foundation (grant no. CRSII5_180258) and the National Competence Center Catalysis (grant no. 51NF40_180544), by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant CATACOAT, no. 758653) and under a Marie Skłodowska-Curie grant agreement no. 945363, by the Swiss Competence Center for Energy Research: Biomass for a Swiss Energy Future through the Swiss Commission for Technology and Innovation (grant no. KTI.2014.0116) and by EPFL (partly through the Tech4Dev programme). We thank M. Studer from the Bern University of Applied Sciences (Switzerland) for providing various wood samples, IP-Suisse for providing corn cob samples, C. Wegmann for compositional analyses of the biomass feedstocks, M. Hannebelle and A. Gagliardi for assisting with the vacuum forming, F. Fadaei Tirani for crystal structure determination, S. Bertella for assistance with graphic design and illustrations, L. Blanchard for assistance with TGA measurements and A. Magrez for powder XRD measurements.
Author information
Authors and Affiliations
Contributions
L.P.M. and J.S.L. conceived of the project and designed the research. L.P.M. performed most of the experiments and drafting of the manuscript. G.R.D. performed most of the biomass fractionation and lignin upgrading experiments and helped design syntheses. A.D. performed most of the material characterization experiments and was supervised by Y.L. and V.M. M.A.H., with the assistance of C.R., performed the catalyst optimization and some of the polymerization, purification and material characterization experiments. M.J.J. performed the life-cycle analysis, aided with the technoeconomic analysis and was supervised by F.M. T.R. performed the pretreatment experiments on the corn cob feedstock. I.S. performed gel permeation chromatography experiments supervised by A.P. M.V. performed injection moulding and tensile strength testing of the PAX samples. H.-A.K. helped design some of the polymerization and characterization experiments. All authors contributed to editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare the following competing financial interests. L.P.M., G.R.D. and J.S.L. are inventors on a European patent application (EP19203000.5) on methods for producing the renewable monomer and polymer described here. G.R.D. and J.S.L. are inventors on a European patent application (EP19202957.7) on methods for producing fragments of lignin with functional groups. J.S.L. is an inventor on a European patent application (EP16165180.7) on methods for producing lignin monomers from lignocellulosic biomass during biomass depolymerization. J.S.L. is a co-founder, and M.A.H. a shareholder, of Bloom Biorenewables Ltd, which is exploring commercial opportunities for aldehyde-stabilized lignin and aldehyde-protected xyloses. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Photos and process yields for production of PAX from commercial xylose.
Alternatively, production steps 3–5 can be replaced by directly precipitating DMGX from the crude esterification reaction (see Extended Data Fig. 4).
Extended Data Fig. 2 Characterization of the distilled DMGX (4-stereoisomer oil) used in polymerizations.
a, 1H NMR spectrum. b, 13C NMR spectrum. c, 2D HSQC NMR spectrum. d, GC chromatogram with percent abundance of each stereoisomer. e, GC-MS spectrum (combination of 4 stereoisomers). f, Photo of DMGX 4-stereoisomer oil. NMR spectrums were acquired in DMSO-d6 at 25 °C.
Extended Data Fig. 3 Characterization of the crystalline DMGX single isomer used in polymerizations.
a, 1H NMR spectrum. b, 13C NMR spectrum. c, 2D HSQC NMR spectrum. d, Crystal structure with probability ellipsoids. The structure was submitted to the Cambridge Crystallographic Data Centre (CCDC) with deposition number CCDC 2121378. e, DSC curve of crystalline DMGX (melting point was determined by maximum of the 1st derivative of DSC curve). f, Photo of DMGX single isomer crystals. NMR spectrums acquired in DMSO-d6 at 25 °C.
Extended Data Fig. 4 Recovery of DMGX via direct precipitation from the crude esterification mixture.
a, 1H NMR spectrum of the precipitate taken in DMSO-d6 at 25 °C. b, HSQC NMR spectrum of the precipitate taken in DMSO-d6 at 25 °C. c, Photo of DMGX precipitating from esterification mixture after chilling the mixture on ice. d, Photo of the precipitate after filtration from the mixture.
Extended Data Fig. 5 DMGX and PAX derived from lignocellulosic biomass.
a, 2D HSQC NMR of distilled DMGX produced from birch wood taken in DMSO-d6 at 25 °C. b, 2D HSQC NMR of crystalline DMGX single isomer produced from birch wood taken in CDCl3 at 25 °C. c, 2D HSQC NMR of 1S-PPTX produced using DMGX from birch wood taken in CDCl3 at 25 °C. d, DMA curves of 1S-PPTX polymers derived from xylose and from birch wood. Tan delta curves are dashed lines.
Extended Data Fig. 6 Minimum selling price of DMGX produced from commercial xylose under various economic scenarios.
Black dashed lines are mean prices for purified terephthalic acid and PLA grade lactic acid and shaded grey areas represent price ranges. IRR is internal rate of return.
Extended Data Fig. 7 Global warming potential of DGMX with various carbon sourcing scenarios.
(left) Using fossil based glyoxylic acid and natural gas heat and power, (middle) using glyoxylic acid from CO2 and natural gas heat and power, and (right) glyoxylic acid from CO2 with biomass burned for heat and power. The red dotted line corresponds to our calculated GWP of commercially available fossil-based purified terephthalic acid.
Extended Data Fig. 8 Supplemental properties of PAX polymers.
a, DMA curves for PAX polymers and PLA with heating ramps applied at 3 °C/min from -50 to 200 °C (shown from 50 °C). Tan delta curves are dashed lines. b, TGA curves with heating rate of 10 °C/min under nitrogen. Incomplete degradation of PAX polymers is attributed to the cyclic nature of the DMGX monomer which likely leads to formation of non-volatile chars in pyrolysis conditions. c, Stress-strain curves of injection-moulded PAX dog bone samples with PLA (NatureWorks 4032D) and PET (Terez 3200) curves for comparison. The displayed curves are from single representative samples but reported tensile data values are all averages of 3–5 samples. d, Full version of panel C with tabulated tensile values. e, DMA curves for pentanediol-based polymers with heating ramps applied at 3 °C/min from −50 to 200 °C (shown from 50 °C). Tan delta curves are dashed lines.
Extended Data Fig. 9 Hydrolytic stability of various PAX polymers in pH 7 phosphate buffer at 37 °C.
The hydrolytic studies were performed in the same manner as detailed in the Supplementary Information section 1.7.14. All polymer films had an initial starting number average molecular weight (Mn) of ~20 kDa. These experiments were performed in triplicate. Error bars represent standard errors.
Extended Data Fig. 10 2D HSQC NMR spectra of chemically-recycled monomers and plastic.
a, Recycled DMGX. b, Recycled 1,6-hexanediol. c, Original PHX used for chemical-recycling study. d, The final recycled PHX using the recycled DMGX and 1,6-hexanediol. NMRs were taken in d-DMSO at 25 °C for the monomers and CDCl3 at 25 °C for the polymers.
Supplementary information
Supplementary Information
Materials and Methods, text, Figs. 1–23, Tables 1–20 and references 38–75.
Supplementary Video 1
Timelapse video of tensile strength testing of the 4S-PHX injection-moulded dog bone.
Supplementary Data 1
The .zip file contains the .cif file of the 1S-DMGX monomer (CCDC reference 2121378), a PDF of the CheckCIF routine, and a Word document containing supporting crystallographic data.
Rights and permissions
About this article
Cite this article
Manker, L.P., Dick, G.R., Demongeot, A. et al. Sustainable polyesters via direct functionalization of lignocellulosic sugars. Nat. Chem. 14, 976–984 (2022). https://doi.org/10.1038/s41557-022-00974-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-00974-5
This article is cited by
-
Performance polyamides built on a sustainable carbohydrate core
Nature Sustainability (2024)
-
Polyamides go circular
Nature Sustainability (2024)
-
Acidic graphene organocatalyst for the superior transformation of wastes into high-added-value chemicals
Nature Communications (2023)
-
Three ways to solve the plastics pollution crisis
Nature (2023)
-
One-Step Acid Bleachable Pretreatment with a Recyclable Acid Hydrotrope and Chlorate for Biomass Valorization
BioEnergy Research (2023)