Abstract
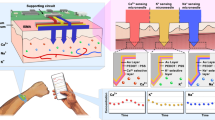
Implementations of wearable microneedle-based arrays of sensors for the monitoring of multiple biomarkers in interstitial fluid have lacked system integration and evidence of robust analytical performance. Here we report the development and testing of a fully integrated wearable array of microneedles for the wireless and continuous real-time sensing of two metabolites (lactate and glucose, or alcohol and glucose) in the interstitial fluid of volunteers performing common daily activities. The device works with a custom smartphone app for data capture and visualization, comprises reusable electronics and a disposable microneedle array, and is optimized for system integration, cost-effective fabrication via advanced micromachining, easier assembly, biocompatibility, pain-free skin penetration and enhanced sensitivity. Single-analyte and dual-analyte measurements correlated well with the corresponding gold-standard measurements in blood or breath. Further validation of the technology in large populations with concurrent validation of sensor readouts through centralized laboratory tests should determine the robustness and utility of real-time simultaneous monitoring of several biomarkers in interstitial fluid.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. The raw and analysed datasets generated during the study are too large to be publicly shared, yet they are available for research purposes from the corresponding authors on reasonable request. Source data are provided with this paper.
Code availability
The firmware for the electronic hardware, custom-developed and written in C, and the source code for the app, custom-developed in Swift, are available from the authors for research purposes on reasonable request.
References
Kim, J., Campbell, A. S., de Ávila, B. E. F. & Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 37, 389–406 (2019).
Dunn, J., Runge, R. & Snyder, M. Wearables and the medical revolution. Per. Med. 15, 429–448 (2018).
Gao, W. et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529, 509–514 (2016).
Heikenfeld, J. et al. Wearable sensors: modalities, challenges, and prospects. Lab Chip 18, 217–248 (2018).
Choi, J. et al. Bio-integrated wearable systems: a comprehensive review. Chem. Rev. 119, 5461–5533 (2019).
Koh, A. et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 8, 366ra165 (2016).
Baker, L. B. et al. Skin-interfaced microfluidic system with personalized sweating rate and sweat chloride analytics for sports science applications. Sci. Adv. 6, eabe3929 (2021).
Emaminejad, S. et al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl Acad. Sci. USA 114, 4625–4630 (2017).
Bandodkar, A. J. et al. Battery-free, skin-interfaced microfluidic/electronic systems for simultaneous electrochemical, colorimetric, and volumetric analysis of sweat. Sci. Adv. 5, eaav3294 (2019).
Lee, H. et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 11, 566–572 (2016).
Imani, S. et al. A wearable chemical–electrophysiological hybrid biosensing system for real-time health and fitness monitoring. Nat. Commun. 7, 11650 (2016).
Bandodkar, A. J. et al. Tattoo-based noninvasive glucose monitoring: a proof-of-concept study. Anal. Chem. 87, 394–398 (2015).
Lipani, L. et al. Non-invasive, transdermal, path-selective and specific glucose monitoring via a graphene-based platform. Nat. Nanotechnol. 13, 504–511 (2018).
Brothers, M. C. et al. Achievements and challenges for real-time sensing of analytes in sweat within wearable platforms. Acc. Chem. Res. 52, 297–306 (2019).
Heikenfeld, J. et al. Accessing analytes in biofluids for peripheral biochemical monitoring. Nat. Biotechnol. 37, 407–419 (2019).
Nyein, H. Y. Y. et al. Regional and correlative sweat analysis using high-throughput microfluidic sensing patches toward decoding sweat. Sci. Adv. 5, eaaw9906 (2019).
Wiorek, A., Parrilla, M., Cuartero, M. & Crespo, G. A. Epidermal patch with glucose biosensor: pH and temperature correction toward more accurate sweat analysis during sport practice. Anal. Chem. 92, 10153–10161 (2020).
Fairbairn, C. E. & Kang, D. Temporal dynamics of transdermal alcohol concentration measured via new-generation wrist-worn biosensor. Alcohol. Clin. Exp. Res. 43, 2060–2069 (2019).
Samant, P. P. et al. Sampling interstitial fluid from human skin using a microneedle patch. Sci. Transl. Med. 12, eaaw0285 (2020).
Tran, B. Q. et al. Proteomic characterization of dermal interstitial fluid extracted using a novel microneedle-assisted technique. J. Proteome Res. 17, 479–485 (2018).
Teymourian, H., Tehrani, F., Mahato, K. & Wang, J. Lab under the skin: microneedle based wearable devices. Adv. Healthc. Mater. 10, 2002255 (2021).
Kolluru, C., Williams, M., Chae, J. & Prausnitz, M. R. Recruitment and collection of dermal interstitial fluid using a microneedle patch. Adv. Healthc. Mater. 8, 1801262 (2019).
Lee, I., Probst, D., Klonoff, D. & Sode, K. Continuous glucose monitoring systems - current status and future perspectives of the flagship technologies in biosensor research. Biosens. Bioelectron. 181, 113054 (2021).
Teymourian, H., Barfidokht, A. & Wang, J. Electrochemical glucose sensors in diabetes management: an updated review (2010–2020). Chem. Soc. Rev. 49, 7671–7709 (2020).
Top 10 Emerging Technologies of 2020 (World Economic Forum, 2020).
Rawson, T. M. et al. Microneedle biosensors for real-time, minimally invasive drug monitoring of phenoxymethylpenicillin: a first-in-human evaluation in healthy volunteers. Lancet Digit. Health 1, e335–e343 (2019).
Smart, W. H. & Subramanian, K. The use of silicon microfabrication technology in painless blood glucose monitoring. Diabetes Technol. Ther. 2, 549–559 (2000).
Gao, J., Huang, W., Chen, Z., Yi, C. & Jiang, L. Simultaneous detection of glucose, uric acid and cholesterol using flexible microneedle electrode array-based biosensor and multi-channel portable electrochemical analyzer. Sens. Actuators B Chem. 287, 102–110 (2019).
Teymourian, H. et al. Microneedle-based detection of ketone bodies along with glucose and lactate: toward real-time continuous interstitial fluid monitoring of diabetic ketosis and ketoacidosis. Anal. Chem. 92, 2291–2300 (2020).
Parrilla, M. et al. Wearable all-solid-state potentiometric microneedle patch for intradermal potassium detection. Anal. Chem. 91, 1578–1586 (2019).
Goud, K. Y. et al. Wearable electrochemical microneedle sensor for continuous monitoring of levodopa: toward Parkinson management. ACS Sens. 4, 2196–2204 (2019).
Ribet, F., Stemme, G. & Roxhed, N. Real-time intradermal continuous glucose monitoring using a minimally invasive microneedle-based system. Biomed. Microdevices 20, 101 (2018).
Sharma, S., Huang, Z., Rogers, M., Boutelle, M. & Cass, A. E. G. Evaluation of a minimally invasive glucose biosensor for continuous tissue monitoring. Anal. Bioanal. Chem. 408, 8427–8435 (2016).
Sharma, S. et al. A pilot study in humans of microneedle sensor arrays for continuous glucose monitoring. Anal. Methods 10, 2088–2095 (2018).
Dervisevic, M. et al. Transdermal electrochemical monitoring of glucose via high-density silicon microneedle array patch. Adv. Funct. Mater. 32, 2009850 (2022).
Burtis, C. A. & Ashwood, E. R. Tietz Textbook of Clinical Chemistry (W.B. Saunders, 1999).
Yu, G. et al. Utility of the early lactate area score as a prognostic marker for septic shock patients in the emergency department. Acute Crit. Care 34, 126–132 (2019).
Sakaguchi, K. et al. Glucose area under the curve during oral glucose tolerance test as an index of glucose intolerance. Diabetol. Int. 7, 53–58 (2016).
Vinson, D. C., Reidinger, C. & Wilcosky, T. Factors affecting the validity of a Timeline Follow-Back interview. J. Stud. Alcohol 64, 733–740 (2003).
Gibb, K. A., Yee, A. S., Johnston, C. C., Martin, S. D. & Nowak, R. M. Accuracy and usefulness of a breath alcohol analyzer. Ann. Emerg. Med. 13, 516–520 (1984).
Campbell, A. S., Kim, J. & Wang, J. Wearable electrochemical alcohol biosensors. Curr. Opin. Electrochem. 10, 126–135 (2018).
Karns-Wright, T. E. et al. Time delays in transdermal alcohol concentrations relative to breath alcohol concentrations. Alcohol Alcohol. 52, 35–41 (2017).
Sakai, J. T., Mikulich-Gilbertson, S. K., Long, R. J. & Crowley, T. J. Validity of transdermal alcohol monitoring: fixed and self-regulated dosing. Alcohol. Clin. Exp. Res. 30, 26–33 (2006).
Wolkowicz, K. L. et al. A review of biomarkers in the context of type 1 diabetes: biological sensing for enhanced glucose control. Bioeng. Transl. Med. 6, e10201 (2021).
Breton, M. D. et al. Addingheart rate signal to a control-to-range artificial pancreas system improves the protection against hypoglycemia during exercise in type 1 diabetes. Diabetes Technol. Ther. 16, 506–511 (2014).
Peyser, T., Dassau, E., Breton, M. & Skyler, J. S. The artificial pancreas: current status and future prospects in the management of diabetes. Ann. N. Y. Acad. Sci. 1311, 102–123 (2014).
Koppes, L. L. J., Dekker, J. M., Hendriks, H. F. J., Bouter, L. M. & Heine, R. J. Moderate alcohol consumption lowers the risk of type 2 diabetes. Diabetes Care 28, 719–725 (2005).
Jones, T. E. et al. Plasma lactate as a marker of metabolic health: implications of elevated lactate for impairment of aerobic metabolism in the metabolic syndrome. Surgery 166, 861–866 (2019).
Wolf, A. et al. Evaluation of continuous lactate monitoring systems within a heparinized in vivo porcine model intravenously and subcutaneously. Biosensors 8, 122 (2018).
Elflein, J. Statista. Diabetes - Statistics & Facts (2021); https://www.statista.com/topics/1723/diabetes/#dossierKeyfigures
Jung, K. A Wearable Alcohol Biosensor (National Institute on Alcohol Abuse and Alcoholism,); (accessed November 15, 2021) https://www.niaaa.nih.gov/wearable-alcohol-biosensor
Acknowledgements
This work is supported by the Center for Wearable Sensors (CWS) at the University of California San Diego and the NIH National Institute of Neurological Disorders and Stroke (Grant Number R21 NS114764 - 01A1). We thank W. Shipley for his support in mechanical characterization of this study.
Author information
Authors and Affiliations
Contributions
F.T., H.T., B.W. and J.K. contributed equally to this work. F.T., H.T., B.W. and J.K. conceived the original project, designed and performed experiments, analysed data, and participated in the figure design and writing of the manuscript. R.P. contributed to the app design and development; A.F., R.A., P.W., N.H. and Z.P. performed the integrated device and sensor fabrications; H.H.-T. contributed to the mechanical stability experiments; C.B. contributed to the electronics design and development; F.Z. and Z.L. performed the cytotoxicity and biocompatibility studies; K.M., A.B. and L.Y. performed the sensor preparations and characterizations. J.W. and P.P.M. conceived the original project, designed the experiments, analysed data, participated in the figure design and manuscript writing, and provided guidance to the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Pawan Jolly, Nicolas Voelcker and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures, tables and video captions.
Supplementary Video 1
Wearable device assembly and its placement on the arm of a participant.
Supplementary Video 2
Demonstration of the sensor app.
Source data
SD for Figs. 3,4
Source data for Figs. 3 and 4.
Rights and permissions
About this article
Cite this article
Tehrani, F., Teymourian, H., Wuerstle, B. et al. An integrated wearable microneedle array for the continuous monitoring of multiple biomarkers in interstitial fluid. Nat. Biomed. Eng 6, 1214–1224 (2022). https://doi.org/10.1038/s41551-022-00887-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-022-00887-1
This article is cited by
-
A wireless battery-free eye modulation patch for high myopia therapy
Nature Communications (2024)
-
Ultrasensitive detection of vital biomolecules based on a multi-purpose BioMEMS for Point of care testing: digoxin measurement as a case study
Scientific Reports (2024)
-
Long-term monitoring of ultratrace nucleic acids using tetrahedral nanostructure-based NgAgo on wearable microneedles
Nature Communications (2024)
-
Interstitial fluid-based wearable biosensors for minimally invasive healthcare and biomedical applications
Communications Materials (2024)
-
Pursuing precision in medicine and nutrition: the rise of electrochemical biosensing at the molecular level
Analytical and Bioanalytical Chemistry (2024)