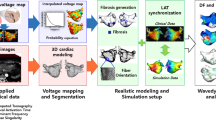
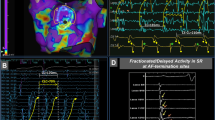
Abstract
Atrial fibrillation (AF)—the most common arrhythmia—significantly increases the risk of stroke and heart failure. Although catheter ablation can restore normal heart rhythms, patients with persistent AF who develop atrial fibrosis often undergo multiple failed ablations, and thus increased procedural risks. Here, we present personalized computational modelling for the reliable predetermination of ablation targets, which are then used to guide the ablation procedure in patients with persistent AF and atrial fibrosis. First, we show that a computational model of the atria of patients identifies fibrotic tissue that, if ablated, will not sustain AF. Then, we report the results of integrating the target ablation sites in a clinical mapping system and testing its feasibility in ten patients with persistent AF. The computational prediction of ablation targets avoids lengthy electrical mapping and could improve the accuracy and efficacy of targeted AF ablation in patients while eliminating the need for repeat procedures.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Code availability
The image-processing software ITK-SNAP is freely available from http://www.itksnap.org/. Computational meshes were generated using the commercial software Simpleware ScanIP (Synopsys). Source code for the human atrial ionic model is freely available from the repository CellML (https://models.physiomeproject.org/exposure/0e03bbe01606be5811691f9d5de10b65). All simulations were conducted using the software package CARP, a free version of which can be downloaded for academic use via https://carp.medunigraz.at/carputils/. Simulation results were visualized using either Meshalyzer (which can be downloaded via https://github.com/cardiosolv/meshalyzer) or ParaView (Kitware) (which can be downloaded via https://www.paraview.org/download/). Data from clinical procedures were visualized using the commercial software CARTOMERGE (Biosense Webster).
Data availability
Relevant data, including patient MRI scans, are available from the authors on approval from the Johns Hopkins Institutional Review Board.
References
Andrade, J., Khairy, P., Dobrev, D. & Nattel, S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ. Res. 114, 1453–1468 (2014).
Go, A. S. et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. J. Am. Med. Assoc. 285, 2370–2375 (2001).
Dorian, P. et al. The impairment of health-related quality of life in patients with intermittent atrial fibrillation: implications for the assessment of investigational therapy. J. Am. Coll. Cardiol. 36, 1303–1309 (2000).
Kalantarian, S., Stern, T. A., Mansour, M. & Ruskin, J. N. Cognitive impairment associated with atrial fibrillation: a meta-analysis. Ann. Intern. Med. 158, 338–346 (2013).
Stewart, S., Murphy, N. F., Walker, A., McGuire, A. & McMurray, J. J. Cost of an emerging epidemic: an economic analysis of atrial fibrillation in the UK. Heart 90, 286–292 (2004).
Calkins, H. et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 14, e275–e444 (2017).
Oakes, R. S. et al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation 119, 1758–1767 (2009).
Marrouche, N. F. et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. J. Am. Med. Assoc. 311, 498–506 (2014).
Scherr, D. et al. Five-year outcome of catheter ablation of persistent atrial fibrillation using termination of atrial fibrillation as a procedural endpoint. Circ. Arrhythm. Electrophysiol. 8, 18–24 (2015).
Xu, J. et al. Atrial extracellular matrix remodeling and the maintenance of atrial fibrillation. Circulation 109, 363–368 (2004).
Tanaka, K. et al. Spatial distribution of fibrosis governs fibrillation wave dynamics in the posterior left atrium during heart failure. Circ. Res. 101, 839–847 (2007).
Verma, A. et al. Approaches to catheter ablation for persistent atrial fibrillation. N. Engl. J. Med. 372, 1812–1822 (2015).
Rappel, W. J., Zaman, J. A. & Narayan, S. M. Mechanisms for the termination of atrial fibrillation by localized ablation: computational and clinical studies. Circ. Arrhythm. Electrophysiol. 8, 1325–1333 (2015).
Haissaguerre, M. et al. Driver domains in persistent atrial fibrillation. Circulation 130, 530–538 (2014).
Swarup, V. et al. Stability of rotors and focal sources for human atrial fibrillation: focal impulse and rotor mapping (FIRM) of AF sources and fibrillatory conduction. J. Cardiovasc. Electrophysiol. 25, 1284–1292 (2014).
Trayanova, N. A. Mathematical approaches to understanding and imaging atrial fibrillation: significance for mechanisms and management. Circ. Res. 114, 1516–1531 (2014).
Zahid, S. et al. Patient-derived models link re-entrant driver localization in atrial fibrillation to fibrosis spatial pattern. Cardiovasc. Res. 110, 443–454 (2016).
Zahid, S. et al. Feasibility of using patient-specific models and the “minimum cut” algorithm to predict optimal ablation targets for left atrial flutter. Heart Rhythm 13, 1687–1698 (2016).
Shim, J. et al. Virtual in-silico modeling guided catheter ablation predicts effective linear ablation lesion set for longstanding persistent atrial fibrillation: multicenter prospective randomized study. Front. Physiol. 8, 792 (2017).
Deng, D. et al. Sensitivity of reentrant driver localization to electrophysiological parameter variability in image-based computational models of persistent atrial fibrillation sustained by a fibrotic substrate. Chaos 27, 093932 (2017).
Hakim, J. B., Murphy, M. J., Trayanova, N. A. & Boyle, P. M. Arrhythmia dynamics in computational models of the atria following virtual ablation of re-entrant drivers. Europace 20, iii45–iii54 (2018).
Narayan, S. M. et al. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J. Am. Coll. Cardiol. 60, 628–636 (2012).
Ramanathan, C., Ghanem, R. N., Jia, P., Ryu, K. & Rudy, Y. Noninvasive electrocardiographic imaging for cardiac electrophysiology and arrhythmia. Nat. Med. 10, 422–428 (2004).
Boyle, P. M. et al. The fibrotic substrate in persistent atrial fibrillation patients: comparison between predictions from computational modeling and measurements from focal impulse and rotor mapping. Front. Physiol. 9, 1151 (2018).
Boyle, P. M. et al. Comparing reentrant drivers predicted by image-based computational modeling and mapped by electrocardiographic imaging in persistent atrial fibrillation. Front. Physiol. 9, 414 (2018).
Lalani, G. G. et al. Organized sources are spatially conserved in recurrent compared to pre-ablation atrial fibrillation: further evidence for non-random electrical substrates. J. Cardiovasc. Electrophysiol. 27, 661–669 (2016).
Brooks, A. G. et al. Outcomes of long-standing persistent atrial fibrillation ablation: a systematic review. Heart Rhythm 7, 835–846 (2010).
McGann, C. et al. Atrial fibrillation ablation outcome is predicted by left atrial remodeling on MRI. Circ. Arrhythm. Electrophysiol. 7, 23–30 (2014).
Khurram, I. M. et al. Magnetic resonance image intensity ratio, a normalized measure to enable interpatient comparability of left atrial fibrosis. Heart Rhythm 11, 85–92 (2014).
Yushkevich, P. A. et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage 31, 1116–1128 (2006).
Karim, R. et al. Evaluation of state-of-the-art segmentation algorithms for left ventricle infarct from late gadolinium enhancement MR images. Med. Image Anal. 30, 95–107 (2016).
Raya, S. P. & Udupa, J. K. Shape-based interpolation of multidimensional objects. IEEE Trans. Med. Imaging 9, 32–42 (1990).
Vadakkumpadan, F. et al. Image-based models of cardiac structure with applications in arrhythmia and defibrillation studies. J. Electro. 42, 157.e1–157.e10 (2009).
Plank, G. et al. From mitochondrial ion channels to arrhythmias in the heart: computational techniques to bridge the spatio-temporal scales. Phil. Trans. A Math. Phys. Eng. Sci. 366, 3381–3409 (2008).
Niederer, S. A. et al. Verification of cardiac tissue electrophysiology simulators using an N-version benchmark. Phil. Trans. A Math. Phys. Eng. Sci. 369, 4331–4351 (2011).
Beg, M. F., Helm, P. A., McVeigh, E., Miller, M. I. & Winslow, R. L. Computational cardiac anatomy using MRI. Magn. Reson. Med. 52, 1167–1174 (2004).
McDowell, K. S. et al. Methodology for patient-specific modeling of atrial fibrosis as a substrate for atrial fibrillation. J. Electrocardiol. 45, 640–645 (2012).
McDowell, K. S. et al. Mechanistic inquiry into the role of tissue remodeling in fibrotic lesions in human atrial fibrillation. Biophys. J. 104, 2764–2773 (2013).
Labarthe, S. et al. A bilayer model of human atria: mathematical background, construction, and assessment. Europace 16, iv21–iv29 (2014).
Roney, C. H. et al. Modelling methodology of atrial fibrosis affects rotor dynamics and electrograms. Europace 18, iv146–iv155 (2016).
Boyle, P. M., Zahid, S. & Trayanova, N. A. Towards personalized computational modelling of the fibrotic substrate for atrial arrhythmia. Europace 18, iv136–iv145 (2016).
Courtemanche, M., Ramirez, R. J. & Nattel, S. Ionic mechanisms underlying human atrial action potential properties: insights from a mathematical model. Am. J. Physiol. 275, H301–H321 (1998).
Krummen, D. E. et al. Mechanisms of human atrial fibrillation initiation: clinical and computational studies of repolarization restitution and activation latency. Circ. Arrhythm. Electrophysiol. 5, 1149–1159 (2012).
Konings, K. T. et al. High-density mapping of electrically induced atrial fibrillation in humans. Circulation 89, 1665–1680 (1994).
Avila, G., Medina, I. M., Jimenez, E., Elizondo, G. & Aguilar, C. I. Transforming growth factor-β1 decreases cardiac muscle L-type Ca2+ current and charge movement by acting on the Cav1.2 mRNA. Am. J. Physiol. Heart Circ. Physiol. 292, H622–H631 (2007).
Nattel, S., Burstein, B. & Dobrev, D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ. Arrhythm. Electrophysiol. 1, 62–73 (2008).
Corradi, D., Callegari, S., Maestri, R., Benussi, S. & Alfieri, O. Structural remodeling in atrial fibrillation. Nat. Clin. Pract. Cardiovasc. Med. 5, 782–796 (2008).
Pedrotty, D. M., Klinger, R. Y., Kirkton, R. D. & Bursac, N. Cardiac fibroblast paracrine factors alter impulse conduction and ion channel expression of neonatal rat cardiomyocytes. Cardiovasc. Res. 83, 688–697 (2009).
Kakkar, R. & Lee, R. T. Intramyocardial fibroblast myocyte communication. Circ. Res. 106, 47–57 (2010).
Ramos-Mondragon, R., Vega, A. V. & Avila, G. Long-term modulation of Na+ and K+ channels by TGF-β1 in neonatal rat cardiac myocytes. Pflug. Arch. 461, 235–247 (2011).
Li, D., Fareh, S., Leung, T. K. & Nattel, S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation 100, 87–95 (1999).
Burstein, B. et al. Changes in connexin expression and the atrial fibrillation substrate in congestive heart failure. Circ. Res. 105, 1213–1222 (2009).
Cochet, H. et al. Relationship between fibrosis detected on late gadolinium-enhanced cardiac magnetic resonance and re-entrant activity assessed with electrocardiographic imaging in human persistent atrial fibrillation. JACC Clin. Electrophysiol. 4, 17–29 (2018).
Vigmond, E. J., Weber Dos Santos, R., Prassl, A. J., Deo, M. & Plank, G. Solvers for the cardiac bidomain equations. Prog. Biophys. Mol. Biol. 96, 3–18 (2008).
Boyle, P. M., Williams, J. C., Ambrosi, C. M., Entcheva, E. & Trayanova, N. A. A comprehensive multiscale framework for simulating optogenetics in the heart. Nat. Commun. 4, 2370 (2013).
Arevalo, H. J. et al. Arrhythmia risk stratification of patients after myocardial infarction using personalized heart models. Nat. Commun. 7, 11437 (2016).
Eason, J. & Trayanova, N. Phase singularities and termination of spiral wave reentry. J. Cardiovasc. Electrophysiol. 13, 672–679 (2002).
Narayan, S. M., Krummen, D. E. & Rappel, W. J. Clinical mapping approach to diagnose electrical rotors and focal impulse sources for human atrial fibrillation. J. Cardiovasc. Electrophysiol. 23, 447–454 (2012).
Arevalo, H., Plank, G., Helm, P., Halperin, H. & Trayanova, N. Tachycardia in post-infarction hearts: insights from 3D image-based ventricular models. PLoS ONE 8, e68872 (2013).
Tops, L. F. et al. Fusion of multislice computed tomography imaging with three-dimensional electroanatomic mapping to guide radiofrequency catheter ablation procedures. Heart Rhythm 2, 1076–1081 (2005).
Dong, J. et al. Impact of heart rhythm status on registration accuracy of the left atrium for catheter ablation of atrial fibrillation. J. Cardiovasc. Electrophysiol. 18, 1269–1276 (2007).
Martinek, M., Nesser, H. J., Aichinger, J., Boehm, G. & Purerfellner, H. Impact of integration of multislice computed tomography imaging into three-dimensional electroanatomic mapping on clinical outcomes, safety, and efficacy using radiofrequency ablation for atrial fibrillation. Pacing Clin. Electrophysiol. 30, 1215–1223 (2007).
Bertaglia, E. et al. Image integration increases efficacy of paroxysmal atrial fibrillation catheter ablation: results from the CartoMerge Italian Registry. Europace 11, 1004–1010 (2009).
Acknowledgements
This project was supported by grants from the NIH (DP1-HL123271 to N.A.T. and U01-HL141074 to N.A.T. and P.M.B.), the AHA (16-SDG-30440006 to P.M.B.), Biosense Webster (to S.N.) and the NSF (graduate fellowship to S.Z.). This project has received funding from the Leducq Foundation (Research Grant number 16 CVD 02). This project was also supported by the Roz and Marvin H. Weiner and Family Foundation, the Dr Francis P. Chiaramonte Private Foundation, M. Poindexter, C. Poindexter and the Norbert and Louise Grunwald Cardiac Arrhythmia Research Fund.
Author information
Authors and Affiliations
Contributions
T.Z., P.M.B., S.Z., A.P., S.N., H.C. and N.A.T. designed the study. P.M.B., S.Z., R.L.A., D.D., W.H.F., J.B.H. and M.J.M. conducted the simulations and analysed the simulation results. T.Z., P.M.B., S.Z., R.L.A., J.B.H., A.P., S.L.Z., H.A., J.E.M., A.K., S.N., D.D.S., H.C. and N.A.T. interpreted the imaging and simulation data. H.A., J.E.M. and H.C. performed the catheter ablation procedures. T.Z., P.M.B. and N.A.T. wrote the paper. H.A., J.E.M., D.D.S. and H.C. edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
N.A.T. has filed a patent application (US patent application number US0161100A1; World Intellectual Property Organization application number WO2015/084876A1; European Patent Office application number EP3076869A4; Japan application number JP2016540570A; Israel application number IL245988D0; entitled ‘Systems and methods for atrial fibrillation treatment and risk assessment’) that is currently under review.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures, tables and video captions.
Supplementary Video 1
Dynamic illustration of all of the steps in the OPTIMA approach.
Supplementary Video 2
Dynamic illustration of the results shown in the top two rows of Fig. 2 (patient 5).
Supplementary Video 3
Same as Supplementary Video 2, but for the results shown in the third and fourth rows of Fig. 2 (patient 7).
Supplementary Video 4
Same as Supplementary Video 2, but for the results shown in the bottom two rows of Fig. 2 (patient 9).
Rights and permissions
About this article
Cite this article
Boyle, P.M., Zghaib, T., Zahid, S. et al. Computationally guided personalized targeted ablation of persistent atrial fibrillation. Nat Biomed Eng 3, 870–879 (2019). https://doi.org/10.1038/s41551-019-0437-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-019-0437-9
This article is cited by
-
Digital twins in medicine
Nature Computational Science (2024)
-
Digital twins for cardiac electrophysiology: state of the art and future challenges
Herzschrittmachertherapie + Elektrophysiologie (2024)
-
2024 European Heart Rhythm Association/Heart Rhythm Society/Asia Pacific Heart Rhythm Society/Latin American Heart Rhythm Society expert consensus statement on catheter and surgical ablation of atrial fibrillation
Journal of Interventional Cardiac Electrophysiology (2024)
-
A possible new cardiac heterogeneity as an arrhythmogenic driver
Scientific Reports (2023)
-
The simplified Kirchhoff network model (SKNM): a cell-based reaction–diffusion model of excitable tissue
Scientific Reports (2023)