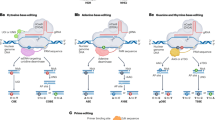
Abstract
Maintenance of genome integrity is an essential process in all organisms. Mechanisms avoiding the formation of DNA lesions or mutations are well described in animals because of their relevance to human health and cancer. In plants, they are of growing interest because DNA damage accumulation is increasingly recognized as one of the consequences of stress. Although the cellular response to DNA damage is mostly studied in response to genotoxic treatments, the main source of DNA lesions is cellular activity itself. This can occur through the production of reactive oxygen species as well as DNA processing mechanisms such as DNA replication or transcription and chromatin dynamics. In addition, how lesions are formed and repaired is greatly influenced by chromatin features and dynamics and by DNA and RNA metabolism. Notably, actively transcribed regions or replicating DNA, because they are less condensed and are sites of DNA processing, are more exposed to DNA damage. However, at the same time, a wealth of cellular mechanisms cooperate to favour DNA repair at these genomic loci. These intricate relationships that shape the distribution of mutations along the genome have been studied extensively in animals but much less in plants. In this Review, we summarize how chromatin dynamics influence lesion formation and DNA repair in plants, providing a comprehensive view of current knowledge and highlighting open questions with regard to what is known in other organisms.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Tubbs, A. & Nussenzweig, A. Endogenous DNA damage as a source of genomic instability in cancer. Cell 168, 644–656 (2017).
Akakpo, R., Carpentier, M.-C., Ie Hsing, Y. & Panaud, O. The impact of transposable elements on the structure, evolution and function of the rice genome. N. Phytol. 226, 44–49 (2020).
Spampinato, C. P. Protecting DNA from errors and damage: an overview of DNA repair mechanisms in plants compared to mammals. Cell. Mol. Life Sci. 74, 1693–1709 (2017).
Röhrig, S. et al. The RecQ-like helicase HRQ1 is involved in DNA crosslink repair in Arabidopsis in a common pathway with the Fanconi anemia-associated nuclease FAN1 and the postreplicative repair ATPase RAD5A. N. Phytol. 218, 1478–1490 (2018).
Dorn, A. et al. An Arabidopsis FANCJ helicase homologue is required for DNA crosslink repair and rDNA repeat stability. PLoS Genet. 15, e1008174 (2019).
Pedroza-Garcia, J.-A., De Veylder, L. & Raynaud, C. Plant DNA polymerases. Int. J. Mol. Sci. 20, 4814 (2019).
Nisa, M.-U., Huang, Y., Benhamed, M. & Raynaud, C. The plant DNA damage response: signaling pathways leading to growth inhibition and putative role in response to stress conditions. Front. Plant Sci. 10, 653 (2019).
Gentric, N., Genschik, P. & Noir, S. Connections between the cell cycle and the DNA damage response in plants. Int. J. Mol. Sci. 22, 9558 (2021).
Pedroza-Garcia, J. A., Xiang, Y. & De Veylder, L. Cell cycle checkpoint control in response to DNA damage by environmental stresses. Plant J. 109, 490–507 (2022).
Herbst, J., Li, Q.-Q. & De Veylder, L. Mechanistic insights into DNA damage recognition and checkpoint control in plants. Nat. Plants https://doi.org/10.1038/s41477-024-01652-9 (2024).
Biedermann, S. et al. The retinoblastoma homolog RBR1 mediates localization of the repair protein RAD51 to DNA lesions in Arabidopsis. EMBO J. 36, 1279–1297 (2017).
Horvath, B. M. et al. Arabidopsis RETINOBLASTOMA RELATED directly regulates DNA damage responses through functions beyond cell cycle control. EMBO J. 36, 1261–1278 (2017).
Nisa, M. et al. Distinctive and complementary roles of E2F transcription factors during plant replication stress responses. Mol. Plant 16, 1269–1282 (2023).
Shen, H. & Li, Z. DNA double-strand break repairs and their application in plant DNA integration. Genes (Basel) 13, 322 (2022).
Ramsden, D. A., Carvajal-Garcia, J. & Gupta, G. P. Mechanism, cellular functions and cancer roles of polymerase-theta-mediated DNA end joining. Nat. Rev. Mol. Cell Biol. 23, 125–140 (2022).
Cortez, D. Replication-coupled DNA repair. Mol. Cell 74, 866–876 (2019).
Ossowski, S. et al. The rate and molecular spectrum of spontaneous mutations in Arabidopsis thaliana. Science 327, 92–94 (2010).
Lindahl, T. & Karlström, O. Heat-induced depyrimidination of deoxyribonucleic acid in neutral solution. Biochemistry 12, 5151–5154 (1973).
Rochette, P. J. et al. Influence of cytosine methylation on ultraviolet-induced cyclobutane pyrimidine dimer formation in genomic DNA. Mutat. Res. 665, 7–13 (2009).
Johann To Berens, P., Golebiewska, K., Peter, J., Staerck, S. & Molinier, J. UV-B-induced modulation of constitutive heterochromatin content in Arabidopsis thaliana. Photochem. Photobiol. Sci. 22, 2153–2166 (2023).
Manova, V. & Gruszka, D. DNA damage and repair in plants—from models to crops. Front. Plant Sci. 6, 885 (2015).
Morales-Ruiz, T. et al. DEMETER and REPRESSOR OF SILENCING 1 encode 5-methylcytosine DNA glycosylases. Proc. Natl Acad. Sci. USA 103, 6853–6858 (2006).
Du, X. et al. Molecular basis of the plant ROS1-mediated active DNA demethylation. Nat. Plants 9, 271–279 (2023).
Li, J. et al. The Arabidopsis ATR–SOG1 signaling module regulates pleiotropic developmental adjustments in response to 3′-blocked DNA repair intermediates. Plant Cell 34, 852–866 (2022).
Kusmartsev, V., Drożdż, M., Schuster-Böckler, B. & Warnecke, T. Cytosine methylation affects the mutability of neighboring nucleotides in germline and soma. Genetics 214, 809–823 (2020).
Shakirov, E. V., Chen, J. J.-L. & Shippen, D. E. Plant telomere biology: the green solution to the end-replication problem. Plant Cell 34, 2492–2504 (2022).
Castillo-González, C., Barbero Barcenilla, B., Young, P. G., Hall, E. & Shippen, D. E. Quantification of 8-oxoG in plant telomeres. Int. J. Mol. Sci. 23, 4990 (2022).
Jia, P., Her, C. & Chai, W. DNA excision repair at telomeres. DNA Repair (Amst.) 36, 137–145 (2015).
Singh, B. N., Sopory, S. K. & Reddy, M. K. Plant DNA topoisomerases: structure, function, and cellular roles in plant development. Crit. Rev. Plant Sci. 23, 251–269 (2004).
Martinez-Garcia, M., White, C. I., Franklin, F. C. H. & Sanchez-Moran, E. The role of topoisomerase II in DNA repair and recombination in Arabidopsis thaliana. Int. J. Mol. Sci. 22, 13115 (2021).
Li, Q. et al. DNA polymerase ε harmonizes topological states and R-loops formation to maintain genome integrity in Arabidopsis. Nat. Commun. 14, 7763 (2023).
Hartung, F. et al. An archaebacterial topoisomerase homolog not present in other eukaryotes is indispensable for cell proliferation of plants. Curr. Biol. 12, 1787–1791 (2002).
Sugimoto-Shirasu, K. et al. RHL1 is an essential component of the plant DNA topoisomerase VI complex and is required for ploidy-dependent cell growth. Proc. Natl Acad. Sci. USA 102, 18736–18741 (2005).
Kirik, V., Schrader, A., Uhrig, J. F. & Hulskamp, M. MIDGET unravels functions of the Arabidopsis topoisomerase VI complex in DNA endoreduplication, chromatin condensation, and transcriptional silencing. Plant Cell 19, 3100–3110 (2007).
Hacker, L., Dorn, A. & Puchta, H. Repair of DNA–protein crosslinks in plants. DNA Repair (Amst.) 87, 102787 (2020).
Hacker, L., Dorn, A., Enderle, J. & Puchta, H. The repair of topoisomerase 2 cleavage complexes in Arabidopsis. Plant Cell 34, 287–301 (2021).
Barbour, A. T. & Wuttke, D. S. RPA-like single-stranded DNA-binding protein complexes including CST serve as specialized processivity factors for polymerases. Curr. Opin. Struct. Biol. 81, 102611 (2023).
Aklilu, B. B., Soderquist, R. S. & Culligan, K. M. Genetic analysis of the Replication Protein A large subunit family in Arabidopsis reveals unique and overlapping roles in DNA repair, meiosis and DNA replication. Nucleic Acids Res. 42, 3104–3118 (2014).
Ragland, R. L., Arlt, M. F., Hughes, E. D., Saunders, T. L. & Glover, T. W. Mice hypomorphic for Atr have increased DNA damage and abnormal checkpoint response. Mamm. Genome 20, 375–385 (2009).
Culligan, K. M., Robertson, C. E., Foreman, J., Doerner, P. & Britt, A. B. ATR and ATM play both distinct and additive roles in response to ionizing radiation. Plant J. 48, 947–961 (2006).
Szurman-Zubrzycka, M. et al. ATR, a DNA damage signaling kinase, is involved in aluminum response in barley. Front. Plant Sci. 10, 1299 (2019).
Pedroza-Garcia, J. A. et al. Maize ATR safeguards genome stability during kernel development to prevent early endosperm endocycle onset and cell death. Plant Cell 33, 2662–2684 (2021).
Kaushal, S. & Freudenreich, C. H. The role of fork stalling and DNA structures in causing chromosome fragility. Genes Chromosomes Cancer 58, 270–283 (2019).
Casper, A. M., Nghiem, P., Arlt, M. F. & Glover, T. W. ATR regulates fragile site stability. Cell 111, 779–789 (2002).
Li, S. & Wu, X. Common fragile sites: protection and repair. Cell Biosci. 10, 29 (2020).
Lee, W. T. C. et al. Single-molecule imaging reveals replication fork coupled formation of G-quadruplex structures hinders local replication stress signaling. Nat. Commun. 12, 2525 (2021).
Williams, S. L. et al. Replication-induced DNA secondary structures drive fork uncoupling and breakage. EMBO J. 42, e114334 (2023).
Cagirici, H. B., Budak, H. & Sen, T. Z. Genome-wide discovery of G-quadruplexes in barley. Sci. Rep. 11, 7876 (2021).
Dvořáčková, M., Fojtová, M. & Fajkus, J. Chromatin dynamics of plant telomeres and ribosomal genes. Plant J. 83, 18–37 (2015).
Huang, M. et al. Plant 45S rDNA clusters are fragile sites and their instability is associated with epigenetic alterations. PLoS ONE 7, e35139 (2012).
Goffová, I. & Fajkus, J. The rDNA loci—intersections of replication, transcription, and repair pathways. Int. J. Mol. Sci. 22, 1302 (2021).
Akamatsu, Y. & Kobayashi, T. The human RNA Polymerase I transcription terminator complex acts as a replication fork barrier that coordinates the progress of replication with rRNA transcription activity. Mol. Cell. Biol. 35, 1871–1881 (2015).
Gadaleta, M. C. & Noguchi, E. Regulation of DNA replication through natural impediments in the eukaryotic genome. Genes (Basel) 8, 98 (2017).
López-Estraño, C., Schvartzman, J. B., Krimer, D. B. & Hernández, P. Characterization of the pea rDNA replication fork barrier: putative cis-acting and trans-acting factors. Plant Mol. Biol. 40, 99–110 (1999).
Mozgová, I., Mokros, P. & Fajkus, J. Dysfunction of chromatin assembly factor 1 induces shortening of telomeres and loss of 45S rDNA in Arabidopsis thaliana. Plant Cell 22, 2768–2780 (2010).
Kaya, H. et al. FASCIATA genes for chromatin assembly factor-1 in Arabidopsis maintain the cellular organization of apical meristems. Cell 104, 131–142 (2001).
Muchová, V. et al. Homology-dependent repair is involved in 45S rDNA loss in plant CAF-1 mutants. Plant J. 81, 198–209 (2015).
Wear, E. E. et al. Genomic analysis of the DNA replication timing program during mitotic S phase in maize (Zea mays) root tips. Plant Cell 29, 2126–2149 (2017).
Concia, L. et al. Genome-wide analysis of the Arabidopsis replication timing program. Plant Physiol. 176, 2166–2185 (2018).
Nisa, M. et al. The plant DNA polymerase theta is essential for the repair of replication-associated DNA damage. Plant J. 106, 1197–1207 (2021).
Canela, A. et al. Genome organization drives chromosome fragility. Cell 170, 507–521.e18 (2017).
Sun, Y. et al. A graph neural network-based interpretable framework reveals a novel DNA fragility-associated chromatin structural unit. Genome Biol. 24, 90 (2023).
Huang, Y. et al. HSFA1a modulates plant heat stress responses and alters the 3D chromatin organization of enhancer–promoter interactions. Nat. Commun. 14, 469 (2023).
Skourti-Stathaki, K. & Proudfoot, N. J. A double-edged sword: R loops as threats to genome integrity and powerful regulators of gene expression. Genes Dev. 28, 1384–1396 (2014).
Hegazy, Y. A., Fernando, C. M. & Tran, E. J. The balancing act of R-loop biology: the good, the bad, and the ugly. J. Biol. Chem. 295, 905–913 (2020).
Petermann, E., Lan, L. & Zou, L. Sources, resolution and physiological relevance of R-loops and RNA–DNA hybrids. Nat. Rev. Mol. Cell Biol. 23, 521–540 (2022).
Yang, Z., Li, M. & Sun, Q. RHON1 co-transcriptionally resolves R-loops for Arabidopsis chloroplast genome maintenance. Cell Rep. 30, 243–256.e5 (2020).
Sun, Q., Csorba, T., Skourti-Stathaki, K., Proudfoot, N. J. & Dean, C. R-loop stabilization represses antisense transcription at the Arabidopsis FLC locus. Science 340, 619–621 (2013).
Gao, J. et al. Toward an understanding of the detection and function of R-loops in plants. J. Exp. Bot. 72, 6110–6122 (2021).
Zhou, J., Zhang, W. & Sun, Q. R-loop: the new genome regulatory element in plants. J. Integr. Plant Biol. 64, 2275–2289 (2022).
Zheng, D., Li, M., Yang, Y., Huang, R. & Zhang, W. R-loops: emerging key regulators in plants. J. Exp. Bot. 74, 2228–2238 (2023).
Li, K. et al. R-loopAtlas: an integrated R-loop resource from 254 plant species sustained by a deep-learning-based tool. Mol. Plant 16, 493–496 (2023).
Yuan, W. et al. ALBA protein complex reads genic R-loops to maintain genome stability in Arabidopsis. Sci. Adv. 5, eaav9040 (2019).
Costantino, L. & Koshland, D. Genome-wide map of R-loop-induced damage reveals how a subset of R-loops contributes to genomic instability. Mol. Cell 71, 487–497.e3 (2018).
Zhou, J. et al. DDM1-mediated R-loop resolution and H2A.Z exclusion facilitates heterochromatin formation in Arabidopsis. Sci. Adv. 9, eadg2699 (2023).
Raskina, O., Shklyar, B. & Nevo, E. The influence of edaphic factors on DNA damage and repair in wild wheat Triticum dicoccoides Körn. (Poaceae, Triticeae). Int. J. Mol. Sci. 24, 6847 (2023).
García-Muse, T. & Aguilera, A. Transcription–replication conflicts: how they occur and how they are resolved. Nat. Rev. Mol. Cell Biol. 17, 553–563 (2016).
St Germain, C., Zhao, H. & Barlow, J. H. Transcription–replication collisions—a series of unfortunate events. Biomolecules 11, 1249 (2021).
Bhowmick, R., Mehta, K. P. M., Lerdrup, M. & Cortez, D. Integrator facilitates RNAPII removal to prevent transcription–replication collisions and genome instability. Mol. Cell 83, 2357–2366.e8 (2023).
Pommier, Y., Nussenzweig, A., Takeda, S. & Austin, C. Human topoisomerases and their roles in genome stability and organization. Nat. Rev. Mol. Cell Biol. 23, 407–427 (2022).
Whittaker, C. & Dean, C. The FLC locus: a platform for discoveries in epigenetics and adaptation. Annu. Rev. Cell Dev. Biol. 33, 555–575 (2017).
Rosa, S., Duncan, S. & Dean, C. Mutually exclusive sense–antisense transcription at FLC facilitates environmentally induced gene repression. Nat. Commun. 7, 13031 (2016).
Fang, X. et al. The 3′ processing of antisense RNAs physically links to chromatin-based transcriptional control. Proc. Natl Acad. Sci. USA 117, 15316–15321 (2020).
Xu, C. et al. R-loop resolution promotes co-transcriptional chromatin silencing. Nat. Commun. 12, 1790 (2021).
Inagaki, S., Takahashi, M., Takashima, K., Oya, S. & Kakutani, T. Chromatin-based mechanisms to coordinate convergent overlapping transcription. Nat. Plants 7, 295–302 (2021).
Anindya, R. Single-stranded DNA damage: protecting the single-stranded DNA from chemical attack. DNA Repair (Amst.) 87, 102804 (2020).
Baxter, C. L., Šviković, S., Sale, J. E., Dean, C. & Costa, S. The intersection of DNA replication with antisense 3′ RNA processing in Arabidopsis FLC chromatin silencing. Proc. Natl Acad. Sci. USA 118, e2107483118 (2021).
Sequeira-Mendes, J. et al. Differences in firing efficiency, chromatin, and transcription underlie the developmental plasticity of the Arabidopsis DNA replication origins. Genome Res. 29, 784–797 (2019).
Borg, M. & Berger, F. Chromatin remodelling during male gametophyte development. Plant J. 83, 177–188 (2015).
van Zanten, M. et al. Seed maturation in Arabidopsis thaliana is characterized by nuclear size reduction and increased chromatin condensation. Proc. Natl Acad. Sci. USA 108, 20219–20224 (2011).
Rathke, C., Baarends, W. M., Awe, S. & Renkawitz-Pohl, R. Chromatin dynamics during spermiogenesis. Biochim. Biophys. Acta Gene Regul. Mech. 1839, 155–168 (2014).
Buttress, T. et al. Histone H2B.8 compacts flowering plant sperm through chromatin phase separation. Nature 611, 614–622 (2022).
Layat, E. et al. The histone chaperone HIRA is a positive regulator of seed germination. Int. J. Mol. Sci. 22, 4031 (2021).
Falk, M., Lukásová, E. & Kozubek, S. Chromatin structure influences the sensitivity of DNA to gamma-radiation. Biochim. Biophys. Acta 1783, 2398–2414 (2008).
Takata, H. et al. Chromatin compaction protects genomic DNA from radiation damage. PLoS ONE 8, e75622 (2013).
Smerdon, M. J., Wyrick, J. J. & Delaney, S. A half century of exploring DNA excision repair in chromatin. J. Biol. Chem. 299, 105118 (2023).
Rocha, L. C., Mittelmann, A., Houben, A. & Techio, V. H. Fragile sites of 45S rDNA of Lolium multiflorum are not hotspots for chromosomal breakages induced by X-ray. Mol. Biol. Rep. 43, 659–665 (2016).
Liu, Q. et al. The histone methyltransferase SUVR2 promotes DSB repair via chromatin remodeling and liquid–liquid phase separation. Mol. Plant 15, 1157–1175 (2022).
Hasegawa, J. et al. Auxin decreases chromatin accessibility through the TIR1/AFBs auxin signaling pathway in proliferative cells. Sci. Rep. 8, 7773 (2018).
Joly, V. & Jacob, Y. Mitotic inheritance of genetic and epigenetic information via the histone H3.1 variant. Curr. Opin. Plant Biol. 75, 102401 (2023).
Jamge, B. et al. Histone variants shape chromatin states in Arabidopsis. eLife 12, RP87714 (2023).
Gao, J. et al. NAP1 family histone chaperones are required for somatic homologous recombination in Arabidopsis. Plant Cell 24, 1437–1447 (2012).
Kolářová, K. et al. Disruption of NAP1 genes in Arabidopsis thaliana suppresses the fas1 mutant phenotype, enhances genome stability and changes chromatin compaction. Plant J. 106, 56–73 (2021).
Monroe, J. G. et al. Mutation bias reflects natural selection in Arabidopsis thaliana. Nature 602, 101–105 (2022).
Staunton, P. M., Peters, A. J. & Seoighe, C. Somatic mutations inferred from RNA-seq data highlight the contribution of replication timing to mutation rate variation in a model plant. Genetics 225, iyad128 (2023).
Quiroz, D., Lensink, M., Kliebenstein, D. J. & Monroe, J. G. Causes of mutation rate variability in plant genomes. Annu. Rev. Plant Biol. 74, 751–775 (2023).
Yan, W., Deng, X. W., Yang, C. & Tang, X. The genome-wide EMS mutagenesis bias correlates with sequence context and chromatin structure in rice. Front. Plant Sci. 12, 579675 (2021).
Sabarinathan, R., Mularoni, L., Deu-Pons, J., Gonzalez-Perez, A. & López-Bigas, N. Nucleotide excision repair is impaired by binding of transcription factors to DNA. Nature 532, 264–267 (2016).
Henry, I. M. et al. Efficient genome-wide detection and cataloging of EMS-induced mutations using exome capture and next-generation sequencing. Plant Cell 26, 1382–1397 (2014).
Krasileva, K. V. et al. Uncovering hidden variation in polyploid wheat. Proc. Natl Acad. Sci. USA 114, E913–E921 (2017).
Belfield, E. J. et al. DNA mismatch repair preferentially protects genes from mutation. Genome Res. 28, 66–74 (2018).
Quiroz, D. et al. The H3K4me1 histone mark recruits DNA repair to functionally constrained genomic regions in plants. Preprint at bioRxiv https://doi.org/10.1101/2022.05.28.493846 (2022).
Lai, J. et al. The transcriptional coactivator ADA2b recruits a structural maintenance protein to double-strand breaks during DNA repair in plants. Plant Physiol. 176, 2613–2622 (2018).
Jiang, J. et al. A diRNA–protein scaffold module mediates SMC5/6 recruitment in plant DNA repair. Plant Cell 34, 3899–3914 (2022).
Jacob, Y. et al. Regulation of heterochromatic DNA replication by histone H3 lysine 27 methyltransferases. Nature 466, 987–991 (2010).
Feng, W. et al. Large-scale heterochromatin remodeling linked to overreplication-associated DNA damage. Proc. Natl Acad. Sci. USA 114, 406–411 (2017).
Davarinejad, H. et al. The histone H3.1 variant regulates TONSOKU-mediated DNA repair during replication. Science 375, 1281–1286 (2022).
Molinier, J., Lechner, E., Dumbliauskas, E. & Genschik, P. Regulation and role of Arabidopsis CUL4–DDB1A–DDB2 in maintaining genome integrity upon UV stress. PLoS Genet. 4, e1000093 (2008).
Schalk, C. et al. DNA DAMAGE BINDING PROTEIN2 shapes the DNA methylation landscape. Plant Cell 28, 2043–2059 (2016).
Córdoba-Cañero, D., Cognat, V., Ariza, R. R., Roldán Arjona, T. & Molinier, J. Dual control of ROS1-mediated active DNA demethylation by DNA damage-binding protein 2 (DDB2). Plant J. 92, 1170–1181 (2017).
Kinner, A., Wu, W., Staudt, C. & Iliakis, G. H2AX in recognition and signaling of DNA double-strand breaks in the context of chromatin. Nucleic Acids Res. 36, 5678–5694 (2008).
Friesner, J. D., Liu, B., Culligan, K. & Britt, A. B. Ionizing radiation-dependent gamma-H2AX focus formation requires ataxia telangiectasia mutated and ataxia telangiectasia mutated and Rad3-related. Mol. Biol. Cell 16, 2566–2576 (2005).
Bourguet, P. et al. The histone variant H2A.W and linker histone H1 co-regulate heterochromatin accessibility and DNA methylation. Nat. Commun. 12, 2683 (2021).
Puizina, J., Siroky, J., Mokros, P., Schweizer, D. & Riha, K. Mre11 deficiency in Arabidopsis is associated with chromosomal instability in somatic cells and Spo11-dependent genome fragmentation during meiosis. Plant Cell 16, 1968–1978 (2004).
Waterworth, W. M. et al. NBS1 is involved in DNA repair and plays a synergistic role with ATM in mediating meiotic homologous recombination in plants. Plant J. 52, 41–52 (2007).
Amiard, S. et al. Distinct roles of the ATR kinase and the Mre11–Rad50–Nbs1 complex in the maintenance of chromosomal stability in Arabidopsis. Plant Cell 22, 3020–3033 (2010).
Fan, T. et al. Arabidopsis γ-H2A.X-INTERACTING PROTEIN participates in DNA damage response and safeguards chromatin stability. Nat. Commun. 13, 7942 (2022).
Lorković, Z. J., Klingenbrunner, M., Cho, C. H. & Berger, F. Identification of plants' functional counterpart of the metazoan mediator of DNA Damage checkpoint 1. EMBO Rep. 25, 1936–1961 (2024).
Arnould, C. et al. Loop extrusion as a mechanism for formation of DNA damage repair foci. Nature 590, 660–665 (2021).
Huang, Y. et al. The matrix revolutions: towards the decoding of the plant chromatin three-dimensional reality. J. Exp. Bot. 71, 5129–5147 (2020).
Liu, C., Cheng, Y.-J., Wang, J.-W. & Weigel, D. Prominent topologically associated domains differentiate global chromatin packing in rice from Arabidopsis. Nat. Plants 3, 742–748 (2017).
Frigerio, C. et al. The chromatin landscape around DNA double-strand breaks in yeast and its influence on DNA repair pathway choice. Int. J. Mol. Sci. 24, 3248 (2023).
Hirakawa, T. et al. LSD1–LIKE1-mediated H3K4me2 demethylation is required for homologous recombination repair. Plant Physiol. 181, 499–509 (2019).
Li, C., Guo, Y., Wang, L. & Yan, S. The SMC5/6 complex recruits the PAF1 complex to facilitate DNA double-strand break repair in Arabidopsis. EMBO J. 42, e112756 (2023).
Roitinger, E. et al. Quantitative phosphoproteomics of the ataxia telangiectasia-mutated (ATM) and ataxia telangiectasia-mutated and rad3-related (ATR) dependent DNA damage response in Arabidopsis thaliana. Mol. Cell. Proteom. 14, 556–571 (2015).
Donà, M. & Mittelsten Scheid, O. DNA damage repair in the context of plant chromatin. Plant Physiol. 168, 1206–1218 (2015).
Campi, M., D’Andrea, L., Emiliani, J. & Casati, P. Participation of chromatin-remodeling proteins in the repair of ultraviolet-B-damaged DNA. Plant Physiol. 158, 981–995 (2012).
Aleksandrov, R., Hristova, R., Stoynov, S. & Gospodinov, A. The chromatin response to double-strand DNA breaks and their repair. Cells 9, 1853 (2020).
Banerjee, S. & Roy, S. An insight into understanding the coupling between homologous recombination mediated DNA repair and chromatin remodeling mechanisms in plant genome: an update. Cell Cycle 20, 1760–1784 (2021).
Shaked, H., Avivi-Ragolsky, N. & Levy, A. A. Involvement of the Arabidopsis SWI2/SNF2 chromatin remodeling gene family in DNA damage response and recombination. Genetics 173, 985–994 (2006).
Jiang, J. et al. A SWI/SNF subunit regulates chromosomal dissociation of structural maintenance complex 5 during DNA repair in plant cells. Proc. Natl Acad. Sci. USA 116, 15288–15296 (2019).
Wu, N. & Yu, H. The Smc complexes in DNA damage response. Cell Biosci. 2, 5 (2012).
Bansbach, C. E., Bétous, R., Lovejoy, C. A., Glick, G. G. & Cortez, D. The annealing helicase SMARCAL1 maintains genome integrity at stalled replication forks. Genes Dev. 23, 2405–2414 (2009).
Zhang, Y. et al. CHR721, interacting with OsRPA1a, is essential for both male and female reproductive development in rice. Plant Mol. Biol. 103, 473–487 (2020).
Zhang, Y., Chen, Q., Zhu, G., Zhang, D. & Liang, W. Chromatin-remodeling factor CHR721 with non-canonical PIP-box interacts with OsPCNA in rice. BMC Plant Biol. 22, 164 (2022).
Lugli, N., Sotiriou, S. K. & Halazonetis, T. D. The role of SMARCAL1 in replication fork stability and telomere maintenance. DNA Repair (Amst.) 56, 129–134 (2017).
Mazin, A. V., Mazina, O. M., Bugreev, D. V. & Rossi, M. J. Rad54, the motor of homologous recombination. DNA Repair (Amst.) 9, 286–302 (2010).
Ceballos, S. J. & Heyer, W.-D. Functions of the Snf2/Swi2 family Rad54 motor protein in homologous recombination. Biochim. Biophys. Acta 1809, 509–523 (2011).
Hirakawa, T., Hasegawa, J., White, C. I. & Matsunaga, S. RAD54 forms DNA repair foci in response to DNA damage in living plant cells. Plant J. 90, 372–382 (2017).
Hirakawa, T. & Matsunaga, S. Characterization of DNA repair foci in root cells of Arabidopsis in response to DNA damage. Front. Plant Sci. 10, 990 (2019).
Hirakawa, T., Katagiri, Y., Ando, T. & Matsunaga, S. DNA double-strand breaks alter the spatial arrangement of homologous loci in plant cells. Sci. Rep. 5, 11058 (2015).
Meschichi, A. et al. The plant-specific DDR factor SOG1 increases chromatin mobility in response to DNA damage. EMBO Rep. 23, e54736 (2022).
Zhang, C. et al. The chromatin-remodeling factor AtINO80 plays crucial roles in genome stability maintenance and in plant development. Plant J. 82, 655–668 (2015).
Kandasamy, M. K., McKinney, E. C., Deal, R. B., Smith, A. P. & Meagher, R. B. Arabidopsis actin-related protein ARP5 in multicellular development and DNA repair. Dev. Biol. 335, 22–32 (2009).
Zhou, W. et al. Distinct roles of the histone chaperones NAP1 and NRP and the chromatin-remodeling factor INO80 in somatic homologous recombination in Arabidopsis thaliana. Plant J. 88, 397–410 (2016).
Rosa, M., Von Harder, M., Cigliano, R. A., Schlögelhofer, P. & Mittelsten Scheid, O. The Arabidopsis SWR1 chromatin-remodeling complex is important for DNA repair, somatic recombination, and meiosis. Plant Cell 25, 1990–2001 (2013).
Zhang, J. et al. The SUMO ligase MMS21 profoundly influences maize development through its impact on genome activity and stability. PLoS Genet. 17, e1009830 (2021).
Xu, P. et al. AtMMS21, an SMC5/6 complex subunit, is involved in stem cell niche maintenance and DNA damage responses in Arabidopsis roots. Plant Physiol. 161, 1755–1768 (2013).
Oztas, O., Selby, C. P., Sancar, A. & Adebali, O. Genome-wide excision repair in Arabidopsis is coupled to transcription and reflects circadian gene expression patterns. Nat. Commun. 9, 1503 (2018).
Selby, C. P., Lindsey-Boltz, L. A., Li, W. & Sancar, A. Molecular mechanisms of transcription-coupled repair. Annu. Rev. Biochem. 92, 115–144 (2023).
Zhang, C. et al. Arabidopsis cockayne syndrome A-like proteins 1A and 1B form a complex with CULLIN4 and damage DNA binding protein 1A and regulate the response to UV irradiation. Plant Cell 22, 2353–2369 (2010).
Kaya, S., Adebali, O., Oztas, O. & Sancar, A. Genome-wide excision repair map of cyclobutane pyrimidine dimers in Arabidopsis and the roles of CSA1 and CSA2 proteins in transcription-coupled repair. Photochem. Photobiol. 98, 707–712 (2022).
Al Khateeb, W. M., Sher, A. A., Marcus, J. M. & Schroeder, D. F. UVSSA, UBP12, and RDO2/TFIIS contribute to Arabidopsis UV tolerance. Front. Plant Sci. 10, 516 (2019).
Schalk, C. et al. Small RNA-mediated repair of UV-induced DNA lesions by the DNA DAMAGE-BINDING PROTEIN 2 and ARGONAUTE 1. Proc. Natl Acad. Sci. USA 114, E2965–E2974 (2017).
Wei, W. et al. A role for small RNAs in DNA double-strand break repair. Cell 149, 101–112 (2012).
Miki, D. et al. Efficient generation of diRNAs requires components in the posttranscriptional gene silencing pathway. Sci. Rep. 7, 301 (2017).
Wang, Q. & Goldstein, M. Small RNAs recruit chromatin-modifying enzymes MMSET and Tip60 to reconfigure damaged DNA upon double-strand break and facilitate repair. Cancer Res. 76, 1904–1915 (2016).
Durut, N. et al. Long noncoding RNAs contribute to DNA damage resistance in Arabidopsis thaliana. Genetics 225, iyad135 (2023).
Herbst, J., Nagy, S. H., Vercauteren, I., De Veylder, L. & Kunze, R. The long non-coding RNA LINDA restrains cellular collapse following DNA damage in Arabidopsis thaliana. Plant J. 116, 1370–1384 (2023).
Durut, N. & Mittelsten Scheid, O. The role of noncoding RNAs in double-strand break repair. Front. Plant Sci. 10, 1155 (2019).
Bourguet, P. et al. DNA polymerase epsilon is required for heterochromatin maintenance in Arabidopsis. Genome Biol. 21, 283 (2020).
Roldán-Arjona, T., Ariza, R. R. & Córdoba-Cañero, D. DNA base excision repair in plants: an unfolding story with familiar and novel characters. Front. Plant Sci. 10, 1055 (2019).
Martínez-Macías, M. I., Córdoba-Cañero, D., Ariza, R. R. & Roldán-Arjona, T. The DNA repair protein XRCC1 functions in the plant DNA demethylation pathway by stimulating cytosine methylation (5-meC) excision, gap tailoring, and DNA ligation. J. Biol. Chem. 288, 5496–5505 (2013).
Córdoba-Cañero, D., Morales-Ruiz, T., Roldán-Arjona, T. & Ariza, R. R. Single-nucleotide and long-patch base excision repair of DNA damage in plants. Plant J. 60, 716–728 (2009).
Acknowledgements
We apologize to authors whose work is related to plant chromatin dynamics and RNA metabolism involved in DNA damage or genome integrity maintenance but could not be cited because of either our oversight or space limitation. This work was supported by a PhD fellowship from the University Paris-Saclay to C.B.-S. and a research grant from Agence Nationale de la Recherche (no. ANR-CE20-0027) to C.R.
Author information
Authors and Affiliations
Contributions
C.R. and C.B.-S. conceptualized the manuscript framework. C.R., C.B.-S. and M.R. wrote the original draft of the manuscript. C.B.-S., C.R. and M.B. produced, reviewed and edited the figures. M.B., D.L. and C.B. reviewed the manuscript. C.B.-S. and C.R. edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bergis-Ser, C., Reji, M., Latrasse, D. et al. Chromatin dynamics and RNA metabolism are double-edged swords for the maintenance of plant genome integrity. Nat. Plants (2024). https://doi.org/10.1038/s41477-024-01678-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41477-024-01678-z