Abstract
Background/Objectives
To determine risk factors and treatment outcomes in dysthyroid optic neuropathy (DON) at a single tertiary ophthalmic centre.
Methods
Retrospective audit of DON patients who have received intravenous methylprednisolone (IVMP) therapy at Royal Victorian Eye and Ear Hospital, Melbourne, Australia from July 2015 to October 2021.
Results
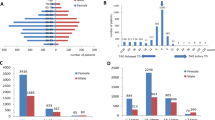
Study included 24 patients (58% female) with an average age of 59.8 ± 14.7 years at DON diagnosis. Majority (92%) had Graves’ hyperthyroidism and 77% had a smoking history. At diagnosis, average visual acuity (VA) of worse eye was LogMAR 0.46, and 48% had relative afferent pupillary defect. Proptosis (89%) and diplopia (73%) were most commonly present at diagnosis. 78% showed predominantly extra-ocular muscle enlargement, and apical crowding (52%) on radiology. 38% (n = 9/24) responded to IVMP alone, 58% (n = 14/24) progressed to surgical orbital decompression. The average total cumulative dose of IVMP during DON treatment was 6.8 ± 1.9 g. 29% required further treatment after IVMP and surgical decompression, 4 (17%) had additional radiotherapy, and three (13%) required immuno-modulatory therapy. Average final VA was LogMAR 0.207, with all patients having inactive TED at final follow-up (mean 1.7 years). In refractory DON cases, 71% retained VA ≥ 6/9 and 48% had DON reversal.
Conclusions
DON patients typically present in late 50s, with a smoking history and predominant extra-ocular muscle enlargement. High-dose IVMP fully resolved DON in only 38%. A considerable proportion required urgent orbital decompression. Most patients retained good vision at final follow-up.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the Royal Victorian Eye and Ear Hospital but restrictions apply to the availability of these data. These data were utilised under a specific license for the current study and are not publicly accessible. Data are however available from the authors upon reasonable request and with permission of the Royal Victorian Eye and Ear Hospital.
References
Agarwal A, Khanam S. Dysthyroid optic neuropathy. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK564299/.
Ahmed SO, Lee AG. Dysthyroid optic neuropathy: a review. Eye Brain. 2011;3:25–36. https://doi.org/10.2147/EB.S23238.
McKeag D, Lane C, Lazarus JH, Baldeschi L, Boboridis K, Dickinson AJ, et al. Clinical features of dysthyroid optic neuropathy: a European Group on Graves’ Orbitopathy (EUGOGO) survey. Br J Ophthalmol. 2007;91:455–8. https://doi.org/10.1136/bjo.2006.094607.
Dayan CM, Dayan MR. Dysthyroid optic neuropathy: a clinical diagnosis or a definable entity? Br J Ophthalmol. 2007;91:409–10.
Andrade A, Faria T, Meneghim R, Hashimoto M, Jorge E, Jorge E. Detection of dysthyroid optic neuropathy by optical coherence tomography. Res Sq. 2022. https://doi.org/10.21203/rs.3.rs-1883360/v1.
Guo J, Li X, Ma R, Gan L, Qian J. The changes of retinal nerve fibre layer and ganglion cell layer with different severity of thyroid eye disease. Eye. 2022;36:129–34. https://doi.org/10.1038/s41433-021-01453-w.
Luo L, Li D, Gao L, Wang W. Retinal nerve fiber layer and ganglion cell complex thickness as a diagnostic tool in early stage dysthyroid optic neuropathy. Eur J Ophthalmol. 2021. https://doi.org/10.1177/11206721211062030.
Dave TV, Laghmisetty S, Krishnamurthy G, Bejjanki K, Ganguly A, Jonnadula GB, et al. Retinal vascularity, nerve fiber, and ganglion cell layer thickness in thyroid eye disease on optical coherence tomography angiography. Orbit. 2022;41:170–7.
Ferreri FM, Ferreri G. Introductory chapter: the dysthyroid optic neuropathy. In: Ferreri FM, editor. Optic Nerve. Rijeka: IntechOpen; 2018. Chapter 1. https://doi.org/10.5772/intechopen.82491.
Blandford AD, Zhang D, Chundury RV, Perry JD. Dysthyroid optic neuropathy: update on pathogenesis, diagnosis, and management. Expert Rev Ophthalmol. 2017;12:111–21. https://doi.org/10.1080/17469899.2017.1276444.
Bartalena L, Kahaly GJ, Baldeschi L, Dayan CM, Eckstein A, Marcocci C, et al. The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur J Endocrinol. 2021;185:G43–67. https://doi.org/10.1530/EJE-21-0479.
Smith TJ, Kahaly GJ, Ezra DG, Fleming JC, Dailey RA, Tang RA, et al. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med. 2017;376:1748–61.
Bartalena L, Krassas GE, Wiersinga WM, Marcocci C, Salvi M, Daumerie C, et al. Efficacy and safety of three different cumulative doses of intravenous methylprednisolone for moderate to severe and active graves’ orbitopathy. J Clin Endocrinol Metab. 2018;103:1702–11. https://doi.org/10.1210/jc.2017-02182.
Mombaerts I, Schiettecatte J, Decallonne B, Velkeniers B. Successful treatment of compressive optic neuropathy with tocilizumab in a patient with thyroid-associated orbitopathy. Eur Thyroid J. 2020;9:163–7. https://doi.org/10.1159/000505343.
Currò N, Covelli D, Vannucchi G, Campi I, Pirola G, Simonetta S, et al. Therapeutic outcomes of high-dose intravenous steroids in the treatment of dysthyroid optic neuropathy. Thyroid. 2014;24:897–905. https://doi.org/10.1089/thy.2013.0445.
Migliori ME, Gladstone GJ. Determination of the normal range of exophthalmometric values for black and white adults. Arch Ophthalmol. 1984;102:981–2. https://doi.org/10.1016/0002-9394(84)90127-2.
Tsai CC, Kau HC, Kao SC, Hsu WM. Exophthalmos of patients with Graves’ disease in Chinese of Taiwan. Eye. 2006;20:569–73. https://doi.org/10.1038/sj.eye.6701925.
Perros P. The thyrotropin receptor and thyroid eye disease. Clin Endocrinol. 1993;38:367–72. https://doi.org/10.1111/j.1365-2265.1993.tb01080.x.
Kendler DL, Lippa J, Rootman J. The initial clinical characteristics of Graves’ orbitopathy vary with age and sex. Arch Ophthalmol. 1993;111:197–201. https://doi.org/10.1001/archopht.1993.01090020051024.
Khong JJ, Finch S, De Silva C, Rylander S, Craig JE, Selva D, et al. Risk factors for graves’ orbitopathy; the Australian thyroid-associated orbitopathy research (ATOR) study. J Clin Endocrinol Metab. 2016;101:2711–20. https://doi.org/10.1210/jc.2015-4294.
Manji N, Carr-Smith JD, Boelaert K, Allahabadia A, Armitage M, Chatterjee VK, et al. Influences of age, gender, smoking, and family history on autoimmune thyroid disease phenotype. J Clin Endocrinol Metab. 2006;91:4873–80. https://doi.org/10.1210/jc.2006-1402.
Wong Y, Dickinson J, Perros P, Dayan C, Veeramani P, Morris D, et al. A British Ophthalmological Surveillance Unit (BOSU) study into dysthyroid optic neuropathy in the United Kingdom. Eye. 2018;32:1555–62. https://doi.org/10.1038/s41433-018-0144-x.
West M, Stranc M. Long-term results of four-wall orbital decompression for Graves’ ophthalmopathy. Br J Plast Surg. 1997;50:507–16.
Fatourechi V, Bergstralh EJ, Garrity JA, Bartley GB, Beatty CW, Offord KP, et al. Predictors of response to transantral orbital decompression in severe Graves’ ophthalmopathy. Mayo Clin Proc. 1994;69:841–8.
Korkmaz S, Konuk O. Surgical treatment of dysthyroid optic neuropathy: long-term visual outcomes with comparison of 2-wall versus 3-wall orbital decompression. Curr Eye Res. 2016;41:159–64.
Liang QW, Yang H, Luo W, He JF, Du Y. Effect of orbital decompression on dysthyroid optic neuropathy: a retrospective case series. Medicine. 2019;98:e14162.
Jeon C, Shin JH, Woo KI, Kim YD. Clinical profile and visual outcomes after treatment in patients with dysthyroid optic neuropathy. Korean J Ophthalmol. 2012;26:73–9. https://doi.org/10.3341/kjo.2012.26.2.73.
Tagami M, Honda S, Azumi A. Preoperative clinical factors and visual outcomes following orbital decompression with dysthyroid optic neuropathy. BMC Ophthalmol. 2020;20:30 https://doi.org/10.1186/s12886-020-1314-8.
Wiersinga WM. Immunosuppressive treatment of Graves’ ophthalmopathy. Thyroid. 1992;2:229–33.
Kemchoknatee P, Tangon D, Srisombut T. A single-center analysis of visual outcomes and associated factors after intravenous methylprednisolone treatment for dysthyroid optic neuropathy. BMC Ophthalmol. 2023;23:32.
Author information
Authors and Affiliations
Contributions
IC was responsible for designing the protocol, writing the manuscript, conducting the search, extracting and analysing data, interpreting results, updating reference lists, and creating tables and figures. JJK was responsible for conceiving the project, designing the protocol, and providing feedback on the report. TH was responsible for reviewing the article and providing feedback on the report.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chung, I.Y., Hardy, T.G. & Khong, J.J. Dysthyroid optic neuropathy: a case series at a tertiary ophthalmic referral centre. Eye 38, 1168–1172 (2024). https://doi.org/10.1038/s41433-023-02856-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02856-7