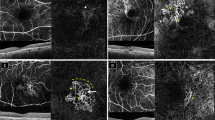
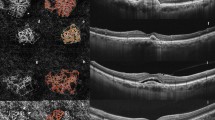
Abstract
Background
To visualize and investigate the three-dimensional (3D) images of macular neovascularization (MNV) in eyes with neovascular age-related macular degeneration using optical coherence tomography angiography (OCTA) according to the treatment response to intravitreal aflibercept injection (IVI).
Methods
OCTA images at baseline and 12 weeks (after three loading IVIs) were retrospectively reconstructed as 3D images for patients with type 1 and 2 MNV treated with the “pro-re-nata” regimen. The fluid-free and persistent fluid groups were divided according to the presence of subretinal and intraretinal fluid at 12 weeks after treatment. Using reconstructed 3D images of MNV, the volume, average volume per slice, and z-axis of the volumetric structure were evaluated.
Results
Twenty-three and nine were classified into the fluid-free and persistent fluid groups, respectively. The MNV volume decreased significantly from baseline to 12 weeks in the fluid-free group (p = 0.005), not in the persistent fluid group (p = 0.250). The average volume of MNV per slice at 12 weeks correlated with the persistent fluid group in both the univariate and multivariate analyses (p = 0.034, p = 0.039, Exp [B] = 14.005).
Conclusions
This study may provide a perspective on vascular volumetric changes of MNV according to treatment response.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Image data extracted (MC) from the HEYEX software (Heidelberg Engineering, Heidelberg, Germany), and clinical information were anonymized by the first author (MC) and securely stored on an encrypted portable device. Raw data, including image data and clinical information, as well as supplementary video files (also anonymized), are available upon request to the first author or corresponding author (MC and SWK).
References
Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–16. https://doi.org/10.1016/s2214-109x(13)70145-1.
Yannuzzi LA, Negrão S, Iida T, Carvalho C, Rodriguez-Coleman H, Slakter J, et al. Retinal angiomatous proliferation in age-related macular degeneration. Retina. 2001;21:416–34. https://doi.org/10.1097/00006982-200110000-00003.
Faatz H, Farecki ML, Rothaus K, Gunnemann F, Gutfleisch M, Lommatzsch A, et al. Optical coherence tomography angiography of types 1 and 2 choroidal neovascularization in age-related macular degeneration during anti-VEGF therapy: evaluation of a new quantitative method. Eye. 2019;33:1466–71. https://doi.org/10.1038/s41433-019-0429-8.
Pilotto E, Frizziero L, Daniele AR, Convento E, Longhin E, Guidolin F, et al. Early OCT angiography changes of type 1 CNV in exudative AMD treated with anti-VEGF. Br J Ophthalmol. 2019;103:67–71. https://doi.org/10.1136/bjophthalmol-2017-311752.
Coscas F, Cabral D, Pereira T, Geraldes C, Narotamo H, Miere A, et al. Quantitative optical coherence tomography angiography biomarkers for neovascular age-related macular degeneration in remission. PLoS ONE. 2018;13:e0205513 https://doi.org/10.1371/journal.pone.0205513.
Choi M, Kim SW, Yun C, Oh J. OCT angiography features of neovascularization as predictive factors for frequent recurrence in age-related macular degeneration. Am J Ophthalmol. 2020;213:109–19. https://doi.org/10.1016/j.ajo.2020.01.012.
Choi M, Kim SW, Yun C, Oh JH, Oh J. Predictive role of optical coherence tomography angiography for exudation recurrence in patients with type 1 neovascular age-related macular degeneration treated with pro-re-nata protocol. Eye. 2023;37:34–41. https://doi.org/10.1038/s41433-021-01879-2.
Choi M, Ahn S, Yun C, Kim SW. Quantitative OCT angiography findings according to pattern classification of type 1 neovascularization exudative age-related macular degeneration. Eye. 2022;36:414–23. https://doi.org/10.1038/s41433-021-01496-z.
Cabral D, Coscas F, Pereira T, Français C, Geraldes C, Laiginhas R, et al. Quantitative optical coherence tomography angiography biomarkers in a treat-and-extend dosing regimen in neovascular age-related macular degeneration. Transl Vis Sci Technol. 2020;9:18. https://doi.org/10.1167/tvst.9.3.18.
Borrelli E, Mastropasqua L, Souied E, Sadda S, Vella G, Toto L, et al. Longitudinal assessment of type 3 macular neovascularization using 3D volume-rendering OCTA. Can J Ophthalmol. 2022;57:228–35. https://doi.org/10.1016/j.jcjo.2021.04.020.
Borrelli E, Sacconi R, Brambati M, Bandello F, Querques G. In vivo rotational three-dimensional OCTA analysis of microaneurysms in the human diabetic retina. Sci Rep. 2019;9:16789. https://doi.org/10.1038/s41598-019-53357-1.
Borrelli E, Sacconi R, Klose G, de Sisternes L, Bandello F, Querques G. Rotational three-dimensional OCTA: a notable new imaging tool to characterize type 3 macular neovascularization. Sci Rep. 2019;9:17053. https://doi.org/10.1038/s41598-019-53307-x.
Borrelli E, Sacconi R, Querques L, Battista M, Bandello F, Querques G. Quantification of diabetic macular ischemia using novel three-dimensional optical coherence tomography angiography metrics. J Biophotonics. 2020;13:e202000152. https://doi.org/10.1002/jbio.202000152.
Spaide RF, Suzuki M, Yannuzzi LA, Matet A, Behar-Cohen F. Volume-rendered angiographic and structural optical coherence tomography angiography of macular telangiectasia type2. Retina. 2017;37:424–35. https://doi.org/10.1097/iae.0000000000001344.
Maloca PM, Spaide RF, de Carvalho ER, Studer HP, Hasler PW, Scholl HPN, et al. Novel biomarker of sphericity and cylindricity indices in volume-rendering optical coherence tomography angiography in normal and diabetic eyes: a preliminary study. Graefes Arch Clin Exp Ophthalmol. 2020;258:711–23. https://doi.org/10.1007/s00417-019-04582-x.
Reich M, Dreesbach M, Boehringer D, Schottenhamml J, Gehring E, Scholl HPN, et al. Negative vessel remodeling in stargardt disease quantified with volume-rendered optical coherence tomography angiography. Retina. 2021;41:1948–57. https://doi.org/10.1097/iae.0000000000003110.
Sekiryu T, Sugano Y, Ojima A, Mori T, Furuta M, Okamoto M, et al. Hybrid three-dimensional visualization of choroidal vasculature imaged by swept-source optical coherence tomography. Transl Vis Sci Technol. 2019;8:31 https://doi.org/10.1167/tvst.8.5.31.
Tsuji S, Sekiryu T, Sugano Y, Ojima A, Kasai A, Okamoto M, et al. Semantic segmentation of the choroid in swept source optical coherence tomography images for volumetrics. Sci Rep. 2020;10:1088 https://doi.org/10.1038/s41598-020-57788-z.
Richard G, Monés J, Wolf S, Korobelnik JF, Guymer R, Goldstein M, et al. Scheduled versus Pro Re Nata Dosing in the VIEW Trials. Ophthalmology. 2015;122:2497–503. https://doi.org/10.1016/j.ophtha.2015.08.014.
Lee WK, Baek J, Dansingani KK, Lee JH, Freund KB. Choroidal morphology in eyes with polypoidal choroidal vasculopathy and normal or subnormal subfoveal choroidal thickness. Retina. 2016;36:S73–82. https://doi.org/10.1097/iae.0000000000001346.
Holmen IC, Konda SM, Pak JW, McDaniel KW, Blodi B, Stepien KE, et al. Prevalence and severity of artifacts in optical coherence tomographic angiograms. JAMA Ophthalmol. 2020;138:119–26. https://doi.org/10.1001/jamaophthalmol.2019.4971.
Kim SW, Oh J, Kwon SS, Yoo J, Huh K. Comparison of choroidal thickness among patients with healthy eyes, early age-related maculopathy, neovascular age-related macular degeneration, central serous chorioretinopathy, and polypoidal choroidal vasculopathy. Retina. 2011;31:1904–11. https://doi.org/10.1097/IAE.0b013e31821801c5.
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–82. https://doi.org/10.1038/nmeth.2019.
Schmid B, Schindelin J, Cardona A, Longair M, Heisenberg M. A high-level 3D visualization API for Java and ImageJ. BMC Bioinformatics. 2010;11:274 https://doi.org/10.1186/1471-2105-11-274.
Spaide RF. Optical coherence tomography angiography signs of vascular abnormalization with antiangiogenic therapy for choroidal neovascularization. Am J Ophthalmol. 2015;160:6–16. https://doi.org/10.1016/j.ajo.2015.04.012.
Korobelnik JF, Souied EH, Oubraham H, Razavi S, Mauget-Faÿsse M, Savel H, et al. Asssessment of early changes in spectral domain-optical coherence tomography after initiation of treatment with intravitreal aflibercept (eylea) over a 12-week period for patients with neovascular age-related macular degeneration: a multicenter French Study (START). Retina. 2021;41:588–94. https://doi.org/10.1097/iae.0000000000002910.
Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–31. https://doi.org/10.1038/nature03952.
la Sala A, Pontecorvo L, Agresta A, Rosano G, Stabile E. Regulation of collateral blood vessel development by the innate and adaptive immune system. Trends Mol Med. 2012;18:494–501. https://doi.org/10.1016/j.molmed.2012.06.007.
Hou X, Du HJ, Zhou J, Hu D, Wang YS, Li X. Role of junctional adhesion molecule-C in the regulation of inner endothelial blood-retinal barrier function. Front Cell Dev Biol. 2021;9:695657. https://doi.org/10.3389/fcell.2021.695657.
Funding
This research was supported in part by the Bio & Medical Technology Development Program of the NRF funded in part by the Korean government, the Ministry of Science and ICT (MSIP) (NRF- 2020R1A2C1005729).
Author information
Authors and Affiliations
Contributions
MC and SWK were involved in the conception and design of the study. MC, SH were involved in the image extraction and data analysis. MC, SH and SWK were involved in data collection and literature research. SWK, CY and JO were involved in the interpretation and critical revision of the article. MC was involved in drafting of the manuscript. MC, SH, SWK, CY and JO were involved in the final approval of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Choi, M., Han, S., Kim, SW. et al. Volume-rendering three-dimensional image analysis of macular neovascularization in age-related macular degeneration. Eye 38, 1125–1132 (2024). https://doi.org/10.1038/s41433-023-02838-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02838-9