Abstract
Objective
To examine event-based glaucoma progression using optical coherence tomography (OCT) and OCT angiography (OCTA).
Methods
In this retrospective study, glaucoma eyes with ≥2-year and 4-visits of OCT/OCTA imaging were included. Peripapillary capillary density (CD) and retinal nerve fibre layer thickness (RNFL) were obtained from 4.5 mm × 4.5 mm optic nerve head (ONH) scans. Event-based OCT/OCTA progression was defined as decreases in ONH measurements exceeding test-retest variability on ≥2 consecutive visits. Visual field (VF) progression was defined as significant VF mean deviation worsening rates on ≥2 consecutive visits. Inter-instrument agreement on progression detection was compared using kappa(κ) statistics.
Results
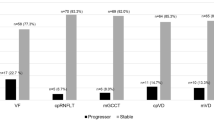
Among 147 eyes (89 participants), OCTA and OCT identified 33(22%) and 25(17%) progressors, respectively. They showed slight agreement (κ = 0.06), with 7(5%) eyes categorized as progressors by both. When incorporating both instruments, the rate of progressors identified increased to 34%. Similar agreement was observed in diagnosis- and severity-stratified analyses (κ < 0.10). Compared to progressors identified only by OCT, progressors identified only by OCTA tended to have thinner baseline RNFL and worse baseline VF. VF progression was identified in 11(7%) eyes. OCT and VF showed fair agreement (κ = 0.26), with 6(4%) eyes categorized as progressors by both. OCTA and VF showed slight agreement (κ = 0.08), with 4(3%) eyes categorized as progressors by both.
Conclusions
OCT and OCTA showed limited agreement on event-based progression detection, with OCT showing better agreement with VF. Both OCT and OCTA detected more progressors than VF. OCT and OCTA may provide valuable, yet different and complementary, information about glaucoma progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. Jama. 2014;311:1901–11.
Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res. 2007;26:688–710.
Kuang TM, Zhang C, Zangwill LM, Weinreb RN, Medeiros FA. Estimating Lead Time Gained by Optical Coherence Tomography in Detecting Glaucoma before Development of Visual Field Defects. Ophthalmology. 2015;122:2002–9.
Medeiros FA, Zangwill LM, Bowd C, Mansouri K, Weinreb RN. The Structure and Function Relationship in Glaucoma: Implications for Detection of Progression and Measurement of Rates of Change. Investig Ophthalmol Vis Sci. 2012;53:6939–46.
Mwanza J-C, Chang RT, Budenz DL, Durbin MK, Gendy MG, Shi W, et al. Reproducibility of Peripapillary Retinal Nerve Fiber Layer Thickness and Optic Nerve Head Parameters Measured with Cirrus HD-OCT in Glaucomatous Eyes. Investig Ophthalmol Vis Sci. 2010;51:5724–30.
González-García AO, Vizzeri G, Bowd C, Medeiros FA, Zangwill LM, Weinreb RN. Reproducibility of RTVue retinal nerve fiber layer thickness and optic disc measurements and agreement with Stratus optical coherence tomography measurements. Am J Ophthalmol. 2009;147:1067–74.1074.e1061.
Wu JH, Moghimi S, Nishida T, Walker E, Kamalipour A, Li E, et al. Evaluation of the long-term variability of macular OCT/OCTA and visual field parameters. Br J Ophthalmol. 2022:bjo-2022-322470. https://doi.org/10.1136/bjo-2022-322470. Epub ahead of print.
Bowd C, Zangwill LM, Weinreb RN, Medeiros FA, Belghith A. Estimating Optical Coherence Tomography Structural Measurement Floors to Improve Detection of Progression in Advanced Glaucoma. Am J Ophthalmol. 2017;175:37–44.
Mwanza JC, Kim HY, Budenz DL, Warren JL, Margolis M, Lawrence SD, et al. Residual and Dynamic Range of Retinal Nerve Fiber Layer Thickness in Glaucoma: Comparison of Three OCT Platforms. Investig Ophthalmol Vis Sci. 2015;56:6344–51.
Yarmohammadi A, Zangwill LM, Diniz-Filho A, Suh MH, Manalastas PI, Fatehee N, et al. Optical Coherence Tomography Angiography Vessel Density in Healthy, Glaucoma Suspect, and Glaucoma Eyes. Investig Ophthalmol Vis Sci. 2016;57:OCT451–9.
Akil H, Huang AS, Francis BA, Sadda SR, Chopra V. Retinal vessel density from optical coherence tomography angiography to differentiate early glaucoma, pre-perimetric glaucoma and normal eyes. PLoS ONE. 2017;12:e0170476.
Rao HL, Pradhan ZS, Weinreb RN, Dasari S, Riyazuddin M, Raveendran S, et al. Relationship of Optic Nerve Structure and Function to Peripapillary Vessel Density Measurements of Optical Coherence Tomography Angiography in Glaucoma. J Glaucoma. 2017;26:548–54.
Lin YH, Huang SM, Yeung L, Ku WC, Chen HS, Lai CC, et al. Correlation of Visual Field With Peripapillary Vessel Density Through Optical Coherence Tomography Angiography in Normal-Tension Glaucoma. Transl Vis Sci Technol. 2020;9:26.
Yarmohammadi A, Zangwill LM, Diniz-Filho A, Suh MH, Yousefi S, Saunders LJ, et al. Relationship between Optical Coherence Tomography Angiography Vessel Density and Severity of Visual Field Loss in Glaucoma. Ophthalmology. 2016;123:2498–508.
Nishida T, Moghimi S, Wu J-H, Chang AC, Diniz-Filho A, Kamalipour A, et al. Association of Initial Optical Coherence Tomography Angiography Vessel Density Loss With Faster Visual Field Loss in Glaucoma. JAMA Ophthalmol. 2022;140:319–26.
Liao MT, Lin MH, Tsai HE, Wu JH, Caffrey JL, Lin JW, et al. Risk stratification and cost-effectiveness analysis of adult patients receiving extracorporeal membrane oxygenation. J Eval Clin Pr. 2022;28:615–23.
Moghimi S, Zangwill LM, Penteado RC, Hasenstab K, Ghahari E, Hou H, et al. Macular and Optic Nerve Head Vessel Density and Progressive Retinal Nerve Fiber Layer Loss in Glaucoma. Ophthalmology. 2018;125:1720–8.
Wu J-H, Moghimi S, Nishida T, Proudfoot JA, Kamalipour A, Zangwill LM et al. Correlation of ganglion cell complex thinning with baseline deep and superficial macular vessel density in glaucoma. Br J Ophthalmol. 2023;107:953–8.
Moghimi S, Bowd C, Zangwill LM, Penteado RC, Hasenstab K, Hou H, et al. Measurement Floors and Dynamic Ranges of OCT and OCT Angiography in Glaucoma. Ophthalmology. 2019;126:980–8.
Nishida T, Moghimi S, Hou H, Proudfoot JA, Chang AC, David RCC, et al. Long-term reproducibility of optical coherence tomography angiography in healthy and stable glaucomatous eyes. Br J Ophthalmol. 2023;107:657–62.
Hou H, Moghimi S, Proudfoot JA, Ghahari E, Penteado RC, Bowd C, et al. Ganglion Cell Complex Thickness and Macular Vessel Density Loss in Primary Open-Angle Glaucoma. Ophthalmology. 2020;127:1043–52.
Shin JW, Song MK, Kook MS. Association Between Progressive Retinal Capillary Density Loss and Visual Field Progression in Open-Angle Glaucoma Patients According to Disease Stage. Am J Ophthalmol. 2021;226:137–47.
Leung CK, Chiu V, Weinreb RN, Liu S, Ye C, Yu M, et al. Evaluation of retinal nerve fiber layer progression in glaucoma: a comparison between spectral-domain and time-domain optical coherence tomography. Ophthalmology. 2011;118:1558–62.
Zhang X, Dastiridou A, Francis BA, Tan O, Varma R, Greenfield DS, et al. Comparison of Glaucoma Progression Detection by Optical Coherence Tomography and Visual Field. Am J Ophthalmol. 2017;184:63–74.
Nguyen AT, Greenfield DS, Bhakta AS, Lee J, Feuer WJ. Detecting Glaucoma Progression Using Guided Progression Analysis with OCT and Visual Field Assessment in Eyes Classified by International Classification of Disease Severity Codes. Ophthalmol Glaucoma. 2019;2:36–46.
Lee A, Sung KR, Shin JW. Progression detection capabilities of circumpapillary and macular vessel density in advanced glaucomatous eyes. Sci Rep. 2022;12:12109.
Rao HL, Kumbar T, Kumar AU, Babu JG, Senthil S, Garudadri CS. Agreement between event-based and trend-based glaucoma progression analyses. Eye (Lond). 2013;27:803–8.
Bussel II, Wollstein G, Schuman JS. OCT for glaucoma diagnosis, screening and detection of glaucoma progression. Br J Ophthalmol. 2014;98:ii15–19.
Aref AA, Budenz DL. Detecting Visual Field Progression. Ophthalmology. 2017;124:S51–6.
Sample PA, Girkin CA, Zangwill LM, Jain S, Racette L, Becerra LM, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Arch Ophthalmol. 2009;127:1136–45.
Girkin CA, Sample PA, Liebmann JM, Jain S, Bowd C, Becerra LM, et al. African Descent and Glaucoma Evaluation Study (ADAGES): II. Ancestry differences in optic disc, retinal nerve fiber layer, and macular structure in healthy subjects. Arch Ophthalmol. 2010;128:541–50.
Jia Y, Tan O, Tokayer J, Potsaid B, Wang Y, Liu JJ, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express. 2012;20:4710–25.
Venugopal JP, Rao HL, Weinreb RN, Pradhan ZS, Dasari S, Riyazuddin M, et al. Repeatability of vessel density measurements of optical coherence tomography angiography in normal and glaucoma eyes. Br J Ophthalmol. 2018;102:352–7.
Vazirani J, Kaushik S, Pandav SS, Gupta P. Reproducibility of retinal nerve fiber layer measurements across the glaucoma spectrum using optical coherence tomography. Indian J Ophthalmol. 2015;63:300–5.
Tan BB, Natividad M, Chua KC, Yip LW. Comparison of retinal nerve fiber layer measurement between 2 spectral domain OCT instruments. J Glaucoma. 2012;21:266–73.
Manalastas PIC, Zangwill LM, Saunders LJ, Mansouri K, Belghith A, Suh MH, et al. Reproducibility of Optical Coherence Tomography Angiography Macular and Optic Nerve Head Vascular Density in Glaucoma and Healthy Eyes. J Glaucoma. 2017;26:851–9.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74.
Gardiner SK, Demirel S. Detecting Change Using Standard Global Perimetric Indices in Glaucoma. Am J Ophthalmol. 2017;176:148–56.
Kamalipour A, Moghimi S, Jacoba CM, Yarmohammadi A, Yeh K, Proudfoot JA, et al. Measurements of OCT Angiography Complement OCT for Diagnosing Early Primary Open-Angle Glaucoma. Ophthalmol Glaucoma. 2022;5:262–74.
Hwang JC, Konduru R, Zhang X, Tan O, Francis BA, Varma R, et al. Relationship among Visual Field, Blood Flow, and Neural Structure Measurements in Glaucoma. Investig Ophthalmol Vis Sci. 2012;53:3020–6.
Shin JW, Lee J, Kwon J, Jo Y, Jeong D, Shon G, et al. Relationship between macular vessel density and central visual field sensitivity at different glaucoma stages. Br J Ophthalmol. 2019;103:1827–33.
Wu J-H, Moghimi S, Nishida T, Mahmoudinezhad G, Zangwill LM, Weinreb RN. Association of macular vessel density and ganglion cell complex thickness with central visual field progression in glaucoma. Br J Ophthalmol. 2022:bjophthalmol-2022-321870. https://doi.org/10.1136/bjo-2022-321870. Epub ahead of print.
Hirasawa K, Smith CA, West ME, Sharpe GP, Shuba LM, Rafuse PE, et al. Discrepancy in Loss of Macular Perfusion Density and Ganglion Cell Layer Thickness in Early Glaucoma. Am J Ophthalmol. 2021;221:39–47.
Leung CK-S, Cheung CYL, Weinreb RN, Qiu K, Liu S, Li H, et al. Evaluation of Retinal Nerve Fiber Layer Progression in Glaucoma: A Study on Optical Coherence Tomography Guided Progression Analysis. Investig Ophthalmol Vis Sci. 2010;51:217–22.
Hou HW, Lin C, Leung CK-S. Integrating Macular Ganglion Cell Inner Plexiform Layer and Parapapillary Retinal Nerve Fiber Layer Measurements to Detect Glaucoma Progression. Ophthalmology. 2018;125:822–31.
Wong D, Chua J, Tan B, Yao X, Chong R, Sng CCA, et al. Combining OCT and OCTA for Focal Structure–Function Modeling in Early Primary Open-Angle Glaucoma. Investig Ophthalmol Vis Sci. 2021;62:8–8.
Sakaguchi K, Higashide T, Udagawa S, Ohkubo S, Sugiyama K. Comparison of Sectoral Structure-Function Relationships in Glaucoma: Vessel Density Versus Thickness in the Peripapillary Retinal Nerve Fiber Layer. Investig Ophthalmol Vis Sci. 2017;58:5251–62.
Funding
This work is supported by National Institutes of Health/National Eye Institute Grants (R01EY034148, R01EY029058, R01EY011008, R01EY019869, R01EY027510, R01EY026574, R01EY018926, P30EY022589); University of California Tobacco Related Disease Research Program (T31IP1511), and an unrestricted grant from Research to Prevent Blindness (New York, NY). The sponsor or funding organization had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
JHW: study design, data analysis, results interpretation, production of tables and figures, and drafting and critical revision of the paper. SM: study design, results interpretation, drafting and critical revision of the paper, and providing research resources and fundings. TN: study design, data analysis, results interpretation, and critical revision of the paper. GM: results interpretation and critical revision of the paper. LZ: subject recruitment, study design, results interpretation, critical revision of the paper, and providing research resources and fundings. RNW: subject recruitment, results interpretation, critical revision of the paper, providing research resources and fundings, and taking full responsibility for the study as the guarantor.
Corresponding author
Ethics declarations
Competing interests
SM reported grants from the National Eye Institute. TN is a consultant of Topcon. LZ reported grants from the National Eye Institute; grants from Heidelberg Engineering and nonfinancial support from Carl Zeiss Meditec, Optovue, Heidelberg Engineering, and Topcon. Consultant of Abbvie, AISight Health and Topcon and patents from Carl Zeiss Meditec. RNW is a consultant of Abbvie, Aerie Pharmaceuticals, Allergan, Amydis, Equinox, Eyenovia, Iantrek, IOPtic, Implandata, Nicox, and Topcon. RNW reported nonfinancial support from Heidelberg Engineering, Carl Zeiss Meditec, Konan Medical, Optovue, Centervue, and Topcon; grants from the National Eye Institute and National Institute of Minority Health Disparities, patents from Toromedes, Carl Zeiss Meditec to UCSD; all outside the submitted work. No other disclosures were reported.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, JH., Moghimi, S., Nishida, T. et al. Detection and agreement of event-based OCT and OCTA analysis for glaucoma progression. Eye 38, 973–979 (2024). https://doi.org/10.1038/s41433-023-02817-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02817-0