Abstract
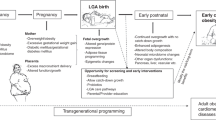
Chronic non-communicable diseases are the leading cause of morbidity and mortality worldwide. Developing and implementing effective preventive strategies is the best way to ensure the overall metabolic health status of the population and to counter the global burden of non-communicable diseases. Predisposition to obesity and other non-communicable diseases is due to a combination of genetic and environmental factors throughout life, but the early environment, particularly the environment during the fetal period and the early years of life, is crucial in determining metabolic health, hence the concept of ‘fetal programming’. The origins of this causal link between environmental factors and disease lie in epigenetic mechanisms. Among the environmental factors, diet plays a crucial role in this process. Substantial evidence documented the key role of macronutrients in the programming of metabolic diseases early in life. Recently, the effect of maternal micronutrient intake on offspring metabolic health in later life emerged. The purpose of this narrative review is to bring to light available evidence in the literature on the effect of maternal micronutrient status on offspring metabolic health and underlying epigenetic mechanisms that drive this link to highlight its potential role in the prevention of non-communicable diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Weihrauch-Blüher S, Schwarz P, Klusmann JH. Childhood obesity: increased risk for cardiometabolic disease and cancer in adulthood. Metabolism. 2019;92:147–52.
Wasniewska M, Pepe G, Aversa T, Bellone S, de Sanctis L, Di Bonito P, et al. Skeptical Look at the Clinical Implication of Metabolic Syndrome in Childhood Obesity. Children. 2023;10:735.
Noncommunicable diseases [Internet]. [cited 2022 Dec 25]. Available from: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases.
Balbus JM, Barouki R, Birnbaum LS, Etzel RA, Gluckman PD, Grandjean P, et al. Early-life prevention of non-communicable diseases. Lancet. 2013;381:3–4.
Oestreich AK, Moley KH. Developmental and transmittable origins of obesity-associated health disorders. Trends Genet. 2017;33:399–407.
Bansal A, Simmons RA. Epigenetics and developmental origins of diabetes: correlation or causation? Am J Physiol Endocrinol Metab. 2018;315:E15–28.
Goyal D, Limesand SW, Goyal R. Epigenetic responses and the developmental origins of health and disease. J Endocrinol. 2019;242:T105–19.
Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429:457–63.
Ling C, Rönn T. Epigenetics in human obesity and Type 2 diabetes. Cell Metab. 2019;29:1028–44.
Ford ND, Behrman JR, Hoddinott JF, Maluccio JA, Martorell R, Ramirez-Zea M, et al. Exposure to improved nutrition from conception to age 2 years and adult cardiometabolic disease risk: a modelling study. Lancet Glob Health. 2018;6:e875–84.
Lumey LH, Khalangot MD, Vaiserman AM. Association between type 2 diabetes and prenatal exposure to the Ukraine famine of 1932-33: a retrospective cohort study. Lancet Diabetes Endocrinol. 2015;3:787–94.
Zhang Q, Sun X, Xiao X, Zheng J, Li M, Yu M, et al. The effect of maternal chromium status on lipid metabolism in female elderly mice offspring and involved molecular mechanism. Biosci Rep. 2017;37:BSR20160362.
Bailey RL, West KP Jr, Black RE. The epidemiology of global micronutrient deficiencies. Ann Nutr Metab. 2015;66:22–33.
Blakstad MM, Fawzi WW, Castro MC, Thompson A, Arabi M, Danaei G. Scaling up prenatal nutrition could reduce the global burden of noncommunicable diseases in the next generation: a modeling analysis. Am J Clin Nutr. 2022;116:1291–302.
Wu Y, Zhang Q, Xiao X. The effect and potential mechanism of maternal micronutrient intake on offspring glucose metabolism: an emerging field. Front Nutr. 2021;8:813.
Venu L, Harishankar N, Krishna TP, Raghunath M. Maternal dietary vitamin restriction increases body fat content but not insulin resistance in WNIN rat offspring up to 6 months of age. Diabetologia. 2004;47:1493–501.
Sinclair KD, Allegrucci C, Singh R, Gardner DS, Sebastian S, Bispham J, et al. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc Natl Acad Sci USA. 2007;104:19351–6.
Saffari A, Shrestha S, Issarapu P, Sajjadi S, Betts M, Sahariah SA, et al. Effect of maternal preconceptional and pregnancy micronutrient interventions on children’s DNA methylation: findings from the EMPHASIS study. Am J Clin Nutr. 2020;112:1099–113.
Takaya J, Yamanouchi S, Kino J, Tanabe Y, Kaneko K. A calcium-deficient diet in dams during gestation increases insulin resistance in male offspring. Nutrients. 2018;10:1745.
Li P, Tang T, Chang X, Fan X, Chen X, Wang R, et al. Abnormality in maternal dietary calcium intake during pregnancy and lactation promotes body weight gain by affecting the gut microbiota in mouse offspring. Mol Nutr Food Res. 2019;63:e1800399.
Morley R, Carlin JB, Dwyer T. Maternal calcium supplementation and cardiovascular risk factors in twin offspring. Int J Epidemiol. 2004;33:1304–9.
Korhonen P, Tihtonen K, Isojärvi J, Ojala R, Ashorn U, Ashorn P, et al. Calcium supplementation during pregnancy and long-term offspring outcome: a systematic literature review and meta-analysis. Ann N. Y Acad Sci. 2022;1510:36–51.
Volpe SL. Magnesium, the metabolic syndrome, insulin resistance, and type 2 diabetes mellitus. Crit Rev Food Sci Nutr. 2008;48:293–300.
Venu L, Kishore YD, Raghunath M. Maternal and perinatal magnesium restriction predisposes rat pups to insulin resistance and glucose intolerance. J Nutr. 2005;135:1353–8.
Venu L, Padmavathi IJN, Kishore YD, Bhanu NV, Rao KR, Sainath PB, et al. Long-term effects of maternal magnesium restriction on adiposity and insulin resistance in rat pups. Obes (Silver Spring). 2008;16:1270–6.
Kostov K. Effects of magnesium deficiency on mechanisms of insulin resistance in Type 2 Diabetes: focusing on the processes of insulin secretion and signaling. Int J Mol Sci. 2019;20:1351.
Martín-Calvo N, Goni L, Tur JA, Martínez JA. Low birth weight and small for gestational age are associated with complications of childhood and adolescence obesity: systematic review and meta-analysis. Obes Rev. 2022;23:e13380.
Takaya J, Kaneko K. Small for gestational age and magnesium in cord blood platelets: intrauterine magnesium deficiency may induce metabolic syndrome in later life. J Pregnancy. 2011;2011:270474.
Huerta MG, Roemmich JN, Kington ML, Bovbjerg VE, Weltman AL, Holmes VF, et al. Magnesium Deficiency Is Associated With Insulin Resistance in Obese Children. Diabetes Care. 2005;28:1175–81.
Nadler JL, Buchanan T, Natarajan R, Antonipillai I, Bergman R, Rude R. Magnesium deficiency produces insulin resistance and increased thromboxane synthesis. Hypertension. 1993;21:1024–9.
Xu L, Li X, Wang X, Xu M. Effects of magnesium supplementation on improving hyperglycemia, hypercholesterolemia, and hypertension in type 2 diabetes: a pooled analysis of 24 randomized controlled trials. Front Nutr. 2023;9:1020327.
Vincent JB. Elucidating a biological role for chromium at a molecular level. Acc Chem Res. 2000;33:503–10.
Mahdi GS. Chromium deficiency might contribute to insulin resistance, Type 2 diabetes mellitus, dyslipidaemia, and atherosclerosis. Diabetic Medicine, (1996). Wiley Online Library. Available from: https://onlinelibrary.wiley.com/doi/10.1002/(SICI)1096-9136(199604)13:4%3C389::AID-DIA65%3E3.0.CO;2-J.
Zhang Q, Xiao X, Zheng J, Li M, Yu M, Ping F, et al. Maternal chromium restriction modulates miRNA profiles related to lipid metabolism disorder in mice offspring. Exp Biol Med (Maywood). 2017;242:1444–52.
Zhang Q, Sun X, Xiao X, Zheng J, Li M, Yu M, et al. Maternal chromium restriction leads to glucose metabolism imbalance in mice offspring through insulin signaling and wnt signaling pathways. Int J Mol Sci. 2016;17:1767.
Suksomboon N, Poolsup N, Yuwanakorn A. Systematic review and meta-analysis of the efficacy and safety of chromium supplementation in diabetes. J Clin Pharm Ther. 2014;39:292–306.
Fukunaka A, Fujitani Y. Role of zinc homeostasis in the pathogenesis of diabetes and obesity. Int J Mol Sci. 2018;19:476.
Olechnowicz J, Tinkov A, Skalny A, Suliburska J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J Physiolog Sci. 2018;68:19.
Jin S, Hu C, Zheng Y. Maternal serum zinc level is associated with risk of preeclampsia: a systematic review and meta-analysis. Front Public Health. 2022;10:968045.
Carducci B, Keats EC, Bhutta ZA. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst Rev. 2021;3:CD000230.
Padmavathi IJN, Kishore YD, Venu L, Ganeshan M, Harishankar N, Giridharan NV, et al. Prenatal and perinatal zinc restriction: effects on body composition, glucose tolerance and insulin response in rat offspring. Exp Physiol. 2009;94:761–9.
Mendes Garrido Abregú F, Gobetto MN, Castañón A, Lucero D, Caniffi C, Elesgaray R, et al. Fetal and postnatal zinc restriction: sex differences in metabolic alterations in adult rats. Nutrition. 2019;65:18–26.
Stewart CP, Christian P, LeClerq SC, West KP, Khatry SK. Antenatal supplementation with folic acid + iron + zinc improves linear growth and reduces peripheral adiposity in school-age children in rural Nepal. Am J Clin Nutr. 2009;90:132–40.
Iannotti LL, Zavaleta N, León Z, Shankar AH, Caulfield LE. Maternal zinc supplementation and growth in Peruvian infants. Am J Clin Nutr. 2008;88:154–60.
Sanusi KO, Ibrahim KG, Abubakar B, Malami I, Bello MB, Imam MU, et al. Effect of maternal zinc deficiency on offspring health: the epigenetic impact. J Trace Elem Med Biol. 2021;65:126731.
Burk RF. Selenium, an antioxidant nutrient. Nutr Clin Care. 2002;5:75–9.
González de Vega R, Fernández-Sánchez ML, Fernández JC, Álvarez Menéndez FV, Sanz-Medel A. Selenium levels and Glutathione peroxidase activity in the plasma of patients with type II diabetes mellitus. J Trace Elem Med Biol. 2016;37:44–9.
WHO recommendations on antenatal care for a positive pregnancy experience [Internet]. [cited 2023 Jan 1]. Available from: https://www.who.int/publications/i/item/9789241549912.
Laureano-Melo R, Império GE, Kluck GEG, da Conceição RR, de Souza JS, Marinho BG, et al. Selenium supplementation during pregnancy and lactation promotes metabolic changes in Wistar rats’ offspring. Clin Exp Pharm Physiol. 2020;47:1272–82.
Ding D, Mou D, Zhao L, Jiang X, Che L, Fang Z, et al. Maternal organic selenium supplementation alleviates LPS induced inflammation, autophagy and ER stress in the thymus and spleen of offspring piglets by improving the expression of selenoproteins. Food Funct. 2021;12:11214–28.
Zeng MS, Li X, Liu Y, Zhao H, Zhou JC, Li K, et al. A high-selenium diet induces insulin resistance in gestating rats and their offspring. Free Radic Biol Med. 2012;52:1335–42.
Li X, Chen H, Epstein PN. Metallothionein and catalase sensitize to diabetes in nonobese diabetic mice. Diabetes. 2006;55:1592–604.
Quezada-Pinedo HG, Cassel F, Duijts L, Muckenthaler MU, Gassmann M, Jaddoe VWV, et al. Maternal iron status in pregnancy and child health outcomes after birth: a systematic review and meta-analysis. Nutrients. 2021;13:2221.
Ferńandez-Real JM, Mcclain D, Review MM. Mechanisms linking glucose homeostasis and iron metabolism toward the onset and progression of Type 2 diabetes. Diabetes Care. 2015;38:2169–76.
Ferrannini E. Insulin resistance, iron, and the liver. Lancet. 2000;355:2181–2.
Vanhees K, Vonhögen IGC, Van Schooten FJ, Godschalk RWL. You are what you eat, and so are your children: the impact of micronutrients on the epigenetic programming of offspring. Cell Mol Life Sci. 2014;71:271–85.
Stewart CP, Christian P, Schulze KJ, Arguello M, LeClerq SC, Khatry SK, et al. Low maternal vitamin B-12 status is associated with offspring insulin resistance regardless of antenatal micronutrient supplementation in rural Nepal. J Nutr. 2011;141:1912–7.
Yajnik CS, Deshpande SS, Jackson AA, Refsum H, Rao S, Fisher DJ, et al. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: the Pune Maternal Nutrition Study. Diabetologia. 2008;51:29–38.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Behere RV, Deshmukh AS, Otiv S, Gupte MD, Yajnik CS. Maternal vitamin B12 status during pregnancy and its association with outcomes of pregnancy and health of the offspring: a systematic review and implications for policy in India. Front Endocrinol (Lausanne). 2021;12:619176.
Honein MA, Paulozzi LJ, Mathews TJ, Erickson JD, Wong LYC. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA. 2001;285:2981–6.
Tanwar VS, Ghosh S, Sati S, Ghose S, Kaur L, Kumar KA, et al. Maternal vitamin B12 deficiency in rats alters DNA methylation in metabolically important genes in their offspring. Mol Cell Biochem. 2020;468:83–96.
Krishnaveni GV, Veena SR, Karat SC, Yajnik CS, Fall CHD. Association between maternal folate concentrations during pregnancy and insulin resistance in Indian children. Diabetologia. 2014;57:110–21.
Lapillonne A. Vitamin D deficiency during pregnancy may impair maternal and fetal outcomes. Med Hypotheses. 2010;74:71–5.
Gallo S, McDermid JM, Al-Nimr RI, Hakeem R, Moreschi JM, Pari-Keener M, et al. Vitamin D supplementation during pregnancy: an evidence analysis center systematic review and meta-analysis. J Acad Nutr Diet. 2020;120:898–924.e4.
Hrudey EJ, Reynolds RM, Oostvogels AJJM, Brouwer IA, Vrijkotte TGM. The association between maternal 25-Hydroxyvitamin D concentration during gestation and early childhood cardio-metabolic outcomes: is there interaction with pre-pregnancy BMI? PLoS One. 2015;10:e0133313.
Zhang H, Chu X, Huang Y, Li G, Wang Y, Li Y, et al. Maternal vitamin D deficiency during pregnancy results in insulin resistance in rat offspring, which is associated with inflammation and Iκbα methylation. Diabetologia. 2014;57:2165–72.
Villa CR, Chen J, Wen B, Sacco SM, Taibi A, Ward WE, et al. Maternal vitamin D beneficially programs metabolic, gut and bone health of mouse male offspring in an obesogenic environment. Int J Obes (Lond). 2016;40:1875–83.
Keller A, Ängquist L, Jacobsen R, Vaag A, Heitmann BL. A retrospective analysis of a societal experiment among the Danish population suggests that exposure to extra doses of vitamin A during fetal development may lower type 2 diabetes mellitus (T2DM) risk later in life. Br J Nutr. 2017;117:731–6.
Christian P, Stewart CP. Maternal micronutrient deficiency, fetal development, and the risk of chronic disease. J Nutr. 2010;140:437–45.
Matthews KA, Rhoten WB, Driscoll HK, Chertow BS. Vitamin A deficiency impairs fetal islet development and causes subsequent glucose intolerance in adult rats. J Nutr. 2004;134:1958–63.
Danielewicz H, Myszczyszyn G, Dębińska A, Myszkal A, Boznański A, Hirnle L. Diet in pregnancy—more than food. Eur J Pediatr. 2017;176:1573–9.
Ambition and Action in Nutrition 2016–2025. Geneva: World Health Organization. 2017;(Licence: CC BY-NC-SA 3.0 IGO.).
Kristensen NB, Madsen ML, Hansen TH, Allin KH, Hoppe C, Fagt S, et al. Intake of macro- and micronutrients in Danish vegans. Nutr J. 2015;14:115.
Popkin BM, Barquera S, Corvalan C, Hofman KJ, Monteiro C, Ng SW, et al. Towards unified and impactful policies to reduce ultra-processed food consumption and promote healthier eating. Lancet Diabetes Endocrinol. 2021;9:462–70.
Lopes SO, Abrantes LCS, Azevedo FM, Morais N, de S, de, Morais D, et al. Food insecurity and micronutrient deficiency in adults: a systematic review and meta-analysis. Nutrients. 2023;15:1074.
Mistry HD, Williams PJ. The importance of antioxidant micronutrients in pregnancy. Oxid Med Cell Longev. 2011;2011:841749.
Franco M, do C, Ponzio BF, Gomes GN, Gil FZ, Tostes R, et al. Micronutrient prenatal supplementation prevents the development of hypertension and vascular endothelial damage induced by intrauterine malnutrition. Life Sci. 2009;85:327–33.
Adams JB, Sorenson JC, Pollard EL, Kirby JK, Audhya T. Evidence-based recommendations for an optimal prenatal supplement for women in the U.S., part two: minerals. Nutrients. 2021;13:1849.
Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, De Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–51.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
AM performed the literature search and wrote the manuscript; AM, RG and CM discussed and edited the manuscript. All authors have read and agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maguolo, A., Gabbianelli, R. & Maffeis, C. Micronutrients in early life and offspring metabolic health programming: a promising target for preventing non-communicable diseases. Eur J Clin Nutr 77, 1105–1112 (2023). https://doi.org/10.1038/s41430-023-01333-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-023-01333-4