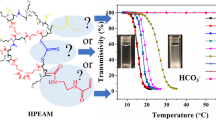
Abstract
A simple method to finely control the thermoresponsive properties of polymers over a wide range of temperatures is to enhance the versatility of the thermoresponsive polymers. One such useful technique is the radical copolymerization of two types of monomers with similar copolymerization reactivities, which allows the hydrophilicity/hydrophobicity balance in the polymer structure to be easily tuned. In this study, we focused on a urethane-containing monomer as the key compound, which can be easily obtained by the reaction between an isocyanate and a hydrophilic precursor monomer containing a hydroxy group. A variety of urethane-embedded acrylamide monomers with different alkyl side chains (ethyl: EtUAAm, n-butyl: BuUAAm, and n-hexyl: HexUAAm) were synthesized from 2-hydroxyethylacrylamide (HEAAm) and alkyl isocyanates. Copolymers of HEAAm and EtUAAm with different compositions were prepared by reversible addition-fragmentation chain transfer (RAFT) polymerization. The obtained copolymer with a high urethane composition (>67%) exhibited a lower critical solution temperature (LCST)-type thermoresponse in water due to the hydrophobic interaction and hydrogen bonding derived from the urethane side groups. The response temperature could be widely varied by altering the composition, molecular weight, end groups and alkyl side chains of the urethane monomer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Alarcón CdlH, Pennadam S, Alexander C. Stimuli responsive polymers for biomedical applications. Chem Soc Rev. 2005;34:276–85.
Schmaljohann D. Thermo- and pH-responsive polymers in drug delivery. Adv Drug Deliv Rev. 2006;58:1655–70.
Cook MT, Haddow P, Kirton SB, McAuley WJ. Polymers Exhibiting Lower Critical Solution Temperatures as a Route to Thermoreversible Gelators for Healthcare. Adv Funct Mater. 2021;31:2008123.
Constantinou AP, Wang L, Wang S, Georgiou TK. Thermoresponsive block copolymers of increasing architecture complexity: a review on structure–property relationships. Polym Chem. 2023;14:223–47.
Bergbreiter DE, Case BL, Liu Y-S, Caraway JW. Poly(N-isopropylacrylamide) Soluble Polymer Supports in Catalysis and Synthesis. Macromolecules. 1998;31:6053–62.
Kanaoka S, Yagi N, Fukuyama Y, Aoshima S, Tsunoyama H, Tsukuda T, et al. Thermosensitive Gold Nanoclusters Stabilized by Well-Defined Vinyl Ether Star Polymers: Reusable and Durable Catalysts for Aerobic Alcohol Oxidation. J Am Chem Soc. 2007;129:12060–1.
Lokuge I, Wang X, Bohn PW. Temperature-Controlled Flow Switching in Nanocapillary Array Membranes Mediated by Poly(N-isopropylacrylamide) Polymer Brushes Grafted by Atom Transfer Radical Polymerization. Langmuir. 2007;23:305–11.
Zhang M, Vora A, Han W, Wojtecki RJ, Maune H, Le ABA, et al. Dual-Responsive Hydrogels for Direct-Write 3D Printing. Macromolecules. 2015;48:6482–8.
Halperin A, Kröger M, Winnik FM. Poly(N-isopropylacrylamide) Phase Diagrams: Fifty Years of Research. Angew Chem Int Ed. 2015;54:15342–67.
Bütün V, Armes SP, Billingham NC. Synthesis and aqueous solution properties of near-monodisperse tertiary amine methacrylate homopolymers and diblock copolymers. Polymer. 2001;42:5993–8.
Xia Y, Burke NAD, Stöver HDH. End Group Effect on the Thermal Response of Narrow-Disperse Poly(N-isopropylacrylamide) Prepared by Atom Transfer Radical Polymerization. Macromolecules. 2006;39:2275–83.
Lang X, Patrick AD, Hammouda B, Hore MJA. Chain terminal group leads to distinct thermoresponsive behaviors of linear PNIPAM and polymer analogs. Polymer. 2018;145:137–47.
Katsumoto Y, Kubosaki N. Tacticity Effects on the Phase Diagram for Poly(N-isopropylacrylamide) in Water. Macromolecules. 2008;41:5955–6.
Nishi K, Hiroi T, Hashimoto K, Fujii K, Han Y-S, Kim T-H, et al. SANS and DLS Study of Tacticity Effects on Hydrophobicity and Phase Separation of Poly(N-isopropylacrylamide). Macromolecules. 2013;46:6225–32.
Hirano T, Miki H, Seno M, Sato T. Effect of polymerization conditions on the syndiotactic-specificity in radical polymerization of N-isopropylacrylamide and fractionation of the obtained polymer according to the stereoregularity. Polymer. 2005;46:5501–5.
Li X, ShamsiJazeyi H, Pesek SL, Agrawal A, Hammouda B, Verduzco R. Thermoresponsive PNIPAAM bottlebrush polymers with tailored side-chain length and end-group structure. Soft Matter. 2014;10:2008–15.
Ida S, Toyama Y, Takeshima S, Kanaoka S. Thermoresponsive core cross-linked star-shaped poly(N-isopropylacrylamide) for reversible and controlled aggregation of nanoscale molecular units. Polym J. 2020;52:359–63.
Sharker KK, Takeshima S, Toyama Y, Ida S, Kanaoka S, Yusa S-i. pH- and thermo-responsive behavior of PNIPAM star containing terminal carboxy groups in aqueous solutions. Polymer. 2020;203:122735.
Terao K, Masahiro A, Nagase M, Takeshima S, Ida S, Kanaoka S. Temperature-Induced Nanostructure Formation Behavior of Core Cross-Linked Star-Shaped Poly(N-isopropylacrylamide) in Water. Macromolecules. 2023;56:5635–41.
Feil H, Bae YH, Feijen J, Kim SW. Effect of Comonomer Hydrophilicity and Ionization on the Lower Critical Solution Temperature of N-isopropylacrylamide Copolymers. Macromolecules. 1993;26:2496–500.
Takei YG, Aoki T, Sanui K, Ogata N, Okano T, Sakurai Y. Temperature-responsive bioconjugates. 2. Molecular design for temperature-modulated bioseparations. Bioconjugate Chem. 1993;4:341–6.
Park J-S, Kataoka K. Precise Control of Lower Critical Solution Temperature of Thermosensitive Poly(2-isopropyl-2-oxazoline) via Gradient Copolymerization with 2-Ethyl-2-oxazoline as a Hydrophilic Comonomer. Macromolecules. 2006;39:6622–30.
Ida S, Kawahara T, Fujita Y, Tanimoto S, Hirokawa Y. Thermoresponsive Properties of Copolymer Gels Induced by Appropriate Hydrophilic/Hydrophobic Balance of Monomer Combination. Macromol Symp. 2015;350:14–21.
Ida S, Nishisako D, Fujiseki A, Kanaoka S. Thermoresponsive properties of polymer hydrogels induced by copolymerization of hydrophilic and hydrophobic monomers: comprehensive study of monomer sequence and water affinity. Soft Matter. 2021;17:6063–72.
Miyazaki H, Kataoka K. Preparation of polyacrylamide derivatives showing thermo-reversible coacervate formation and their potential application to two-phase separation processes. Polymer. 1996;37:681–5.
Sugihara S, Kanaoka S, Aoshima S. Thermosensitive Random Copolymers of Hydrophilic and Hydrophobic Monomers Obtained by Living Cationic Copolymerization1. Macromolecules. 2004;37:1711–9.
Komatsu S, Asoh T-A, Ishihara R, Kikuchi A. Facile preparation of degradable thermoresponsive polymers as biomaterials: Thermoresponsive polymers prepared by radical polymerization degrade to water-soluble oligomers. Polymer. 2017;130:68–73.
Bivigou-Koumba AM, Kristen J, Laschewsky A, Müller-Buschbaum P, Papadakis CM. Synthesis of Symmetrical Triblock Copolymers of Styrene and N-isopropylacrylamide Using Bifunctional Bis(trithiocarbonate)s as RAFT Agents. Macromol Chem Phys. 2009;210:565–78.
Sun B, Lin Y, Wu P, Siesler HW. A FTIR and 2D-IR Spectroscopic Study on the Microdynamics Phase Separation Mechanism of the Poly(N-isopropylacrylamide) Aqueous Solution. Macromolecules. 2008;41:1512–20.
Willcock H, O’Reilly RK. End Group Removal and Modification of RAFT Polymers. Polym Chem. 2010;1:149–57.
Acknowledgements
This work was partially supported by the Japan Society for the Promotion of Science through a Grant-in-Aid for Scientific Research (JP19K05602), for which the authors are grateful.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ida, S., Hashiguchi, R., Murai, Y. et al. Facile synthesis of acrylamide derivative copolymers with side urethane groups for systematic variation of the thermoresponsive behavior. Polym J (2024). https://doi.org/10.1038/s41428-024-00914-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41428-024-00914-9