Abstract
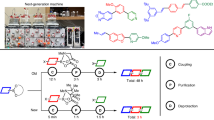
We previously reported the quantitative main-chain scission reaction of polymers by a conjugate substitution reaction in aqueous ammonia, although the detailed behavior of the reaction was not analyzed. Herein, the degradation of a poly(conjugated ester), prepared from 1,4-butylene bis[α-(bromomethyl)acrylate] and fluorescein, with aqueous solution of various nucleophiles was investigated. Direct observation using UV‒vis spectrometry was effective for the analysis at the earliest stage of degradation, while size-exclusion chromatography was used for long-term degradation. The main-chain scissions were observed by a conjugate substitution reaction with ammonia, n-propylamine, diethylamine and α-amino acids, while similar degradation was observed with sodium acetate and water in the presence of triethylamine as a catalyst. The nucleophilicity of the nucleophiles and their affinity for the polymer affected the reaction rates in the earliest stage. On the other hand, to achieve complete chain scission, ammonia was most effective, probably because the dissolution of the degradation products led to the exposure of new polymer surfaces.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Moore CJ. Synthetic polymers in the marine environment: A rapidly increasing, long-term threat. Environ Res. 2008;108:131–9.
Chamas A, Moon H, Zheng J, Qiu Y, Tabassum T, Jang JH, et al. Degradation rates of plastics in the environment. ACS Sustain Chem Eng. 2020;8:3494–511.
Manfra L, Marengo V, Libralato G, Costantini M, De Falco F, Cocca M. Biodegradable polymers: A real opportunity to solve marine plastic pollution? J Hazard Mater. 2021;416:125763.
Suzuki M, Tachibana Y, Kazahaya J, Takizawa R, Muroi F, Kasuya K. Difference in environmental degradability between poly(ethylene succinate) and poly(3-hydroxybutyrate). J Polym Res. 2017;24:217.
Haung Q, Kimura S, Iwata T. Development of self-degradable aliphatic polyesters by embedding lipases via melt extrusion. Polym Degrad Stab. 2021;191:109647.
Suzuki M, Tachibana Y, Soulenthone P, Suzuki T, Takeno H, Kasuya K. Control of marine biodegradation of an aliphatic polyester using endospores. Polym Degrad Stab. 2023;215:110466.
Tsutsuba T, Tachibana Y, Shimizu M, Kasuya K. Marine biodegradation of poly(butylene succinate) incorporating disulfide bonds triggered by a switch function in response to reductive stimuli. ACS Appl Polym Mater. 2023;5:2964–70.
Tachibana Y, Baba T, Kasuya K. Environmental biodegradation control of polymers by cleavage of disulfide bonds. Polym Degrad Stab. 2017;138:18–26.
Uchiyama M, Murakami Y, Satoh K, Kamigaito M. Synthesis and degradation of vinyl polymers with evenly distributed thioacetal bonds in main chains: cationic dt copolymerization of vinyl ethers and cyclic thioacetals. Angew Chem Int Ed. 2022;62:e202215021.
Kamiki R, Kubo T, Satoh K. Addition–fragmentation ring-opening polymerization of bio-based thiocarbonyl l-lactide for dual degradable vinyl copolymers. Macromol Rapid Commun. 2022;44:202200537.
Watanabe H, Kamigaito M. Direct radical copolymerizations of thioamides to generate vinyl polymers with degradable thioether bonds in the backbones. J Am Chem Soc. 2023;145:10948–53.
Kohsaka Y. Conjugate substitution reaction of α-(substituted methyl)acrylates in polymer chemistry. Polym J. 2020;52:1175–83.
Kohsaka Y, Nagai K. Degradable and curable poly(conjugated ester)s prepared by acryl- and conjugate-substitutions of the ‘smallest’ monomer. Eur Polym J. 2020;141:110049.
Kohsaka Y, Nagai K. Controls and effects of monomer junctions and sequences in curable and degradable polyarylate containing acrylate moieties. Macromol Rapid Commun. 2021;42:2000570.
Thompson R, Barclay T, Basu K, Mathias LJ. Facile synthesis and polymerization of ether substituted methacrylates. Polym J. 1995;27:325–38.
Kohsaka Y, Matsumoto Y, Kitayama T. α-(Aminomethyl)acrylate: polymerization and spontaneous post-polymerization modification of β-amino acid ester for a pH/temperature-responsive material. Polym Chem. 2015;6:5026–9.
Kohsaka Y, Nagatsuka N. End-reactive poly(tetrahydrofuran) for functionalization and graft copolymer synthesis via a conjugate substitution reaction. Polym J. 2020;52:75–81.
Habaue S, Shibagaki T, Okamoto Y. Polym J. 1999;31:942–7.
Evans RA, Moad G, Rizzardo E, Thang SH. New free-radical ring-opening acrylate monomers. Macromolecules. 1994;27:7935–7.
Kohsaka Y, Miyazaki T, Hagiwara K. Conjugate substitution and addition of α-substituted acrylate: a highly efficient, facile, convenient, and versatile approach to fabricate degradable polymers. Polym Chem. 2018;9:1610–7.
Kohsaka Y, Yamashita M, Matsuhashi Y, Yamashita S. Synthesis of poly(conjugated ester)s by ring-opening polymerization of cyclic hemiacetal ester bearing acryl skeleton. Eur Polym J. 2019;120:109185.
Zhuang J, Zhao B, Meng X, Schiffman JD, Perry SL, Vachet RW, et al. A programmable chemical switch based on triggerable Michael acceptors. Chem Sci. 2020;11:2103–11.
Xu Y-C, Ren W-M, Zhou H, Gu G-G, Lu X-B. Functionalized polyesters with tunable degradability prepared by controlled ring-opening (co)polymerization of lactones. Macromolecules. 2017;50:3131–42.
Sjöback R, Nygren J, Kubista M. Absorption and fluorescence properties of fluorescein. Spectrochim Acta A Mol Biomol Spectrosc. 1995;51:L7–21.
Goldberg RN, Kishore N, Lennen RM. Thermodynamic Quantities for the Ionization Reactions of buffers. J Phys Chem Ref Data. 2002;31:231–370.
Acknowledgements
This paper is based on results obtained from a project, JPNP18016, commissioned by the New Energy and Industrial Technology Development Organization (NEDO). 1,4-Butylene diacrylate (4) was a kind gift from Osaka Organic Chemical Industry Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Noda, T., Kitagawa, T. & Kohsaka, Y. Degradation of poly(conjugated ester)s using a conjugate substitution reaction with various amines and amino acids in aqueous media. Polym J 56, 343–351 (2024). https://doi.org/10.1038/s41428-023-00859-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-023-00859-5