Abstract
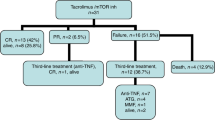
In the early randomized trials the efficacy of calcineurin inhibitors (CNI) in the treatment of graft-versus-host disease (GVHD) was comparable to corticosteroids (CS), but these results became obsolete with the introduction of CNIs in prophylaxis. Recently several effective CNI-free GVHD prophylaxis regimens were introduced based on posttransplantation cyclophosphamide (PTCY) and αβ ex vivo T-cell depletion (αβ-TCD). Among patients treated under these protocols 34 patients with grade II-IV acute (aGVHD) and 40 with moderate and severe chronic (cGVHD) disease were treated with CNIs or other CS-free regimens as the first line. Overall response rate (ORR) was significantly higher in cGVHD than in aGVHD: 80% (95% CI 68–92) vs 47% (95% CI 30–64%), p = 0.0031. In aGVHD it was almost completely restricted to isolated stage III skin GVHD. In cGVHD patients with moderate disease ORR was higher than in severe: 96% (95% CI 88–100%) vs 56% (95%CI 32–81%), p = 0.0022. Two-year overall survival was 76% (95% CI 58–87%) in aGVHD and 95% (95% CI 81–99%) in cGVHD. Failure-free survival was 21% (95% CI 9–37%) in aGVHD and 81% (95% CI 64–91%) in cGVHD. Patients responding to steroid-free regimens had lower use of systemic antibiotics (p = 0.0095), antifungals (p = 0.0319) and antivirals (p < 0.0001).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Primary study data is available through request to Ethical Committee of Pavlov University according to standard operating procedures, e-mail: spbgmutrials@yandex.ru.
References
Doney KC, Weiden PL, Storb R, Thomas ED. Treatment of graft-versus-host disease in human allogeneic marrow graft recipients: a randomized trial comparing antithymocyte globulin and corticosteroids. Am J Hematol. 1981;11:1–8. https://doi.org/10.1002/ajh.2830110102.
Sullivan KM, Shulman HM, Storb R, Weiden PL, Witherspoon RP, McDonald GB, et al. Chronic graft-versus-host disease in 52 patients: adverse natural course and successful treatment with combination immunosuppression. Blood. 1981;57:267–76.
Kennedy MS, Deeg HJ, Storb R, Doney K, Sullivan KM, Witherspoon RP, et al. Treatment of acute graft-versus-host disease after allogeneic marrow transplantation. Randomized study comparing corticosteroids and cyclosporine. Am J Med. 1985;78:978–83. https://doi.org/10.1016/0002-9343(85)90221-9.
Storb R, Deeg HJ, Farewell V, Doney K, Appelbaum F, Beatty P, et al. Marrow transplantation for severe aplastic anemia: methotrexate alone compared with a combination of methotrexate and cyclosporine for prevention of acute graft-versus-host disease. Blood. 1986;68:119–25.
Cragg L, Blazar BR, Defor T, Kolatker N, Miller W, Kersey J, et al. A randomized trial comparing prednisone with antithymocyte globulin/prednisone as an initial systemic therapy for moderately severe acute graft-versus-host disease. Biol Blood Marrow Transplant. 2000;6:441–7. https://doi.org/10.1016/s1083-8791(00)70036-x.
Lee SJ, Zahrieh D, Agura E, MacMillan ML, Maziarz RT, McCarthy PL Jr, et al. Effect of up-front daclizumab when combined with steroids for the treatment of acute graft-versus-host disease: results of a randomized trial. Blood. 2004;104:1559–64. https://doi.org/10.1182/blood-2004-03-0854.
Alousi AM, Weisdorf DJ, Logan BR, Bolaños-Meade J, Carter S, Difronzo N, et al. Blood and Marrow Transplant Clinical Trials Network. Etanercept, mycophenolate, denileukin, or pentostatin plus corticosteroids for acute graft-versus-host disease: a randomized phase 2 trial from the Blood and Marrow Transplant Clinical Trials Network. Blood. 2009;114:511–7. https://doi.org/10.1182/blood-2009-03-212290. Epub 2009 May 14
Bolaños-Meade J, Logan BR, Alousi AM, Antin JH, Barowski K, Carter SL, et al. Phase 3 clinical trial of steroids/mycophenolate mofetil vs steroids/placebo as therapy for acute GVHD: BMT CTN 0802. Blood. 2014;124:3221–7. https://doi.org/10.1182/blood-2014-06-577023.
Sullivan KM, Witherspoon RP, Storb R, Weiden P, Flournoy N, Dahlberg S, et al. Prednisone and azathioprine compared with prednisone and placebo for treatment of chronic graft-v-host disease: prognostic influence of prolonged thrombocytopenia after allogeneic marrow transplantation. Blood. 1988;72:546–54.
Koc S, Leisenring W, Flowers ME, Anasetti C, Deeg HJ, Nash RA, et al. Thalidomide for treatment of patients with chronic graft-versus-host disease. Blood. 2000;96:3995–6.
Arora M, Wagner JE, Davies SM, Blazar BR, Defor T, Enright H, et al. Randomized clinical trial of thalidomide, cyclosporine, and prednisone versus cyclosporine and prednisone as initial therapy for chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2001;7:265–73. https://doi.org/10.1053/bbmt.2001.v7.pm11400948.
Koc S, Leisenring W, Flowers ME, Anasetti C, Deeg HJ, Nash RA, et al. Therapy for chronic graft-versus-host disease: a randomized trial comparing cyclosporine plus prednisone versus prednisone alone. Blood 2002;100:48–51. https://doi.org/10.1182/blood.v100.1.48.
Martin PJ, Storer BE, Rowley SD, Flowers ME, Lee SJ, Carpenter PA, et al. Evaluation of mycophenolate mofetil for initial treatment of chronic graft-versus-host disease. Blood. 2009;113:5074–82. https://doi.org/10.1182/blood-2009-02-202937.
Pidala J, Hamadani M, Dawson P, Martens M, Alousi AM, Jagasia M, et al. Randomized multicenter trial of sirolimus vs prednisone as initial therapy for standard-risk acute GVHD: the BMT CTN 1501 trial. Blood 2020;135:97–107. https://doi.org/10.1182/blood.2019003125.
Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–50.
Luznik L, Bolaños-Meade J, Zahurak M, Chen AR, Smith BD, Brodsky R, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115:3224–30.
Ruggeri A, Labopin M, Bacigalupo A, Afanasyev B, Cornelissen JJ, Elmaagacli A, et al. Post-transplant cyclophosphamide for graft-versus-host disease prophylaxis in HLA matched sibling or matched unrelated donor transplant for patients with acute leukemia, on behalf of ALWP-EBMT. J Hematol Oncol. 2018;11:40. https://doi.org/10.1186/s13045-018-0586-4.
Sanz J, Galimard JE, Labopin M, Afanasyev B, Moiseev I, Angelucci E, et al. Post-transplant cyclophosphamide containing regimens after matched sibling, matched unrelated and haploidentical donor transplants in patients with acute lymphoblastic leukemia in first complete remission, a comparative study of the ALWP of the EBMT. J Hematol Oncol. 2021;14:84. https://doi.org/10.1186/s13045-021-01094-2.
Bolaños-Meade J, Reshef R, Fraser R, Fei M, Abhyankar S, Al-Kadhimi Z, et al. Three prophylaxis regimens (tacrolimus, mycophenolate mofetil, and cyclophosphamide; tacrolimus, methotrexate, and bortezomib; or tacrolimus, methotrexate, and maraviroc) versus tacrolimus and methotrexate for prevention of graft-versus-host disease with haemopoietic cell transplantation with reduced-intensity conditioning: a randomised phase 2 trial with a non-randomised contemporaneous control group (BMT CTN 1203). Lancet Haematol. 2019;6:e132–e143. https://doi.org/10.1016/S2352-3026(18)30221-7.
de Witte MA, Janssen A, Nijssen K, Karaiskaki F, Swanenberg L, van Rhenen A, et al. αβ T-cell graft depletion for allogeneic HSCT in adults with hematological malignancies. Blood Adv 2021;5:240–9. https://doi.org/10.1182/bloodadvances.2020002444.
Shekhovtsova Z, Shelikhova L, Balashov D, Zakharova V, Ilushina M, Voronin K, et al. Control of graft-versus-host disease with rabbit anti-thymocyte globulin, rituximab, and bortezomib in TCRαβ/CD19-depleted graft transplantation for leukemia in children: a single-center retrospective analysis of two GVHD-prophylaxis regimens. Pediatr Transpl. 2020;24:e13594. https://doi.org/10.1111/petr.13594.
Shelikhova L, Glushkova S, Nikolaev R, Dunaikina M, Zhekhovtsova Z, Blagov S, et al. Serotherapy-Free Regimen Improves Non-Relapse Mortality and Immune Recovery Among the Recipients of αβ TCell-Depleted Haploidentical Grafts: Retrospective Study in Childhood Leukemia. Transpl Cell Ther. 2021;27:330.e1–330.e9. https://doi.org/10.1016/j.jtct.2021.01.010.
Morozova EV, Barabanshikova MV, Moiseev IS, Shakirova AI, Barhatov IM, Ushal IE, et al. A prospective pilot study of graft-versus-host disease prophylaxis with post-transplantation cyclophosphamide and ruxolitinib in patients with myelofibrosis. Acta Haematol. 2021;144:158–65. https://doi.org/10.1159/000506758.
Greco R, Lorentino F, Albanese S, Lupo Stanghellini MT, Giglio F, Piemontese S, et al. Posttransplantation cyclophosphamide- and sirolimus-based graft-versus-host-disease prophylaxis in allogeneic stem cell transplant. Transplant Cell Ther. 2021;27:776.e1–776.e13. https://doi.org/10.1016/j.jtct.2021.05.023.
Moiseev IS, Pirogova OV, Alyanski AL, Babenko EV, Gindina TL, Darskaya EI, et al. Risk-adapted GVHD prophylaxis with post-transplantation cyclophosphamide in adults after related, unrelated, and haploidentical transplantations. Eur J Haematol 2018;100:395–402. https://doi.org/10.1111/ejh.13030.
Ruutu T, Gratwohl A, de Witte T, Afanasyev B, Apperley J, Bacigalupo A, et al. Prophylaxis and treatment of GVHD: EBMT-ELN working group recommendations for a standardized practice. Bone Marrow Transplant. 2014;49:168–73.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–8.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–56.
Martin PJ, Bachier CR, Klingemann HG, McCarthy PL, Szabolcs P, Uberti JP, et al. Endpoints for clinical trials testing treatment of acute graft-versus-host disease: a joint statement. Biol Blood Marrow Transplant. 2009;15:777–84.
Lee SJ, Wolff D, Kitko C, Koreth J, Inamoto Y, Jagasia M, et al. Measuring therapeutic response in chronic graft-versus-host disease. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. The 2014 Response Criteria Working Group report. Biol Blood Marrow Transplant. 2015;21:984–99.
Kanakry CG, Ganguly S, Zahurak M, Bolaños-Meade J, Thoburn C, Perkins B, et al. Aldehyde dehydrogenase expression drives human regulatory T cell resistance to posttransplantation cyclophosphamide. Sci Transl Med. 2013;5:211ra157 https://doi.org/10.1126/scitranslmed.3006960.
Ikegawa S, Meguri Y, Mizuhara K, Fukumi T, Kobayashi H, Sumii Y, et al. Pretransplant nivolumab further enhanced Treg expansion after posttransplant cyclophosphamide; another aspect for immune tolerance by PTCy after nivolumab. Leukemia. 2021;35:929–31. https://doi.org/10.1038/s41375-021-01167-8.
Saliba RM, Couriel DR, Giralt S, Rondon G, Okoroji GJ, Rashid A, et al. Prognostic value of response after upfront therapy for acute GVHD. Bone Marrow Transplant. 2012;47:125–31. https://doi.org/10.1038/bmt.2011.41.
Chaleff S, Otto M, Barfield RC, Leimig T, Iyengar R, Martin, et al. A large-scale method for the selective depletion of alphabeta T lymphocytes from PBSC for allogeneic transplantation. Cytotherapy. 2007;9:746–54. https://doi.org/10.1080/14653240701644000.
Luznik L, Pasquini MC, Logan B, Soiffer RJ, Wu J, Devine SM, et al. Randomized Phase III BMT CTN trial of calcineurin inhibitor-free chronic graft-versus-host disease interventions in myeloablative hematopoietic cell transplantation for hematologic malignancies. J Clin Oncol. 2022;40:356–68. https://doi.org/10.1200/JCO.21.02293.
Cooper DL, Manago J, Patel V, Schaar D, Krimmel T, McGrath MK, et al. Incorporation of posttransplant cyclophosphamide as part of standard immunoprophylaxis for all allogeneic transplants: a retrospective, single institution study. Bone Marrow Transplant. 2021;56:1099–105. https://doi.org/10.1038/s41409-020-01144-2.
Bolaños-Meade J, Logan BR, Alousi AM, Antin JH, Barowski K, Carter SL, et al. Phase 3 clinical trial of steroids/mycophenolate mofetil vs steroids/placebo as therapy for acute GVHD: BMT CTN 0802. Blood. 2014;124:3221–335. https://doi.org/10.1182/blood-2014-06-577023.
Franco LM, Gadkari M, Howe KN, Sun J, Kardava L, Kumar P, et al. Immune regulation by glucocorticoids can be linked to cell type-dependent transcriptional responses. J Exp Med. 2019;216:384–406. https://doi.org/10.1084/jem.20180595.
Youssef J, Novosad SA, Winthrop KL. Infection risk and safety of corticosteroid use. Rheum Dis Clin N Am. 2016;42:157-x. https://doi.org/10.1016/j.rdc.2015.08.004.
Cao W, Manicassamy S, Tang H, Kasturi SP, Pirani A, Murthy N, et al. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat Immunol. 2008;9:1157–64. https://doi.org/10.1038/ni.1645.
Mattson E, Xu L, Li L, Liu GE, Xiao Z. Transcriptome profiling of CTLs regulated by rapamycin using RNA-Seq. Immunogenetics. 2014;66:625–33. https://doi.org/10.1007/s00251-014-0790-5.
Zeiser R, Polverelli N, Ram R, Hashmi SK, Chakraverty R, Middeke JM, et al. Ruxolitinib for glucocorticoid-refractory chronic graft-versus-host disease. N Engl J Med. 2021;385:228–38. https://doi.org/10.1056/NEJMoa2033122.
Escamilla Gómez V, García-Gutiérrez V, López Corral L, García Cadenas I, Pérez Martínez A, Márquez Malaver FJ, et al. Ruxolitinib in refractory acute and chronic graft-versus-host disease: a multicenter survey study. Bone Marrow Transplant. 2020;55:641–8. https://doi.org/10.1038/s41409-019-0731-x.
Pidala J, Onstad L, Martin PJ, Hamilton BK, Cutler C, Kitko CL, et al. Initial therapy for chronic graft-versus-host disease: analysis of practice variation and failure-free survival. Blood Adv. 2021;5:4549–59. https://doi.org/10.1182/bloodadvances.2021005286.
Bachier CR, Aggarwal SK, Hennegan K, Milgroom A, Francis K, Dehipawala S, et al. Epidemiology and treatment of chronic graft-versus-host disease post-allogeneic hematopoietic cell transplantation: a US claims analysis. Transplant Cell Ther. 2021;27:504.e1–504.e6. https://doi.org/10.1016/j.jtct.2020.12.027.
Acknowledgements
We acknowledge the significant input in this work and support of this steroid-free approach of our former director, Boris Afanasyev, who passed away in 2020. We thank Michael Maschan for organizing multicenter validation of αβ-TCD prophylaxis.
Funding
This study is supported by Russian Science Fund grant 17-75-20145-P.
Author information
Authors and Affiliations
Contributions
IM: conceptualization, methodology, project administration, writing - original draft, visualization, funding acquisition; MB: formal analysis, writing - original draft, AD, AS, YV: investigation, data curation; EM: methodology, writing - review & editing; SB: methodology, writing - review & editing; AK: supervision, writing - review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moiseev, I., Barabanshikova, M., Dotsenko, A. et al. First-line steroid-free systemic treatment of acute and chronic graft-versus-host disease after novel prophylaxis regimens. Bone Marrow Transplant 58, 257–264 (2023). https://doi.org/10.1038/s41409-022-01879-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-022-01879-0