Abstract
Background
Androgen deprivation therapy (ADT) and androgen receptor signaling inhibitors (ARSI) are associated with deleterious physical effects, which exercise may mitigate; however, exercise has never been studied in patients initiating treatment with ADT and an ARSI. Our objective was to determine whether supervised exercise prior to and during initial therapy could mitigate adverse effects of ADT plus enzalutamide.
Methods
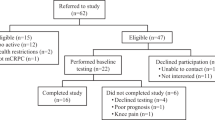
We conducted a single center trial in patients with recurrent prostate cancer treated with ADT and enzalutamide. We randomized 26 patients to 16 weeks of supervised exercise (aerobic and resistance), starting 4 weeks before initiation of ADT and enzalutamide, or usual care. The primary endpoint was change in peak oxygen uptake (VO2peak) as a measure of cardiorespiratory fitness (CRF). Secondary endpoints were functional capacity, maximal strength, body composition, patient-reported outcomes, safety, and feasibility. Analysis of covariance was used to compare outcomes for groups at Week 17 adjusted for baseline values.
Results
The usual care group (N = 13) showed declines from baseline to week 17 in both absolute CRF (−0.31 L/min, −10.9%; p < 0.01) and relative CRF (−3.2 mL/kg/min, −8.9%; p = 0.04); worse fatigue (p = 0.01); and worse quality of life (p = 0.01). At week 17, the exercise group (N = 13) demonstrated improved absolute CRF (between-group change +0.20 L/min, p = 0.05), leg strength (+48.6 kg, p < 0.01) and functional capacity (+21.0 m, p = 0.01) at week 17.
Conclusions
This is the first randomized controlled trial demonstrating a clinically significant decline in CRF in patients initiating ADT and enzalutamide. We show the effectiveness of short-term supervised exercise to mitigate declines in absolute CRF, and improve maximal leg strength and functional capacity.
ClinicalTrials.gov Identifier
NCT02256111
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Nguyen PL, Alibhai SM, Basaria S, D’Amico AV, Kantoff PW, Keating NL, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol. 2015;67:825–36.
James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N. Engl J Med. 2017;377:338–51.
Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N. Engl J Med. 2017;377:352–60.
Chi KN, Agarwal N, Bjartell A, Chung BH, Pereira de Santana Gomes AJ, Given R, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N. Engl J Med. 2019;381:13–24.
Armstrong AJ, Szmulewitz RZ, Petrylak DP, Holzbeierlein J, Villers A, Azad A, et al. ARCHES: A randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37:2974–86.
Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N. Engl J Med. 2019;381:121–31.
Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl J Med. 2012;367:1187–97.
Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl J Med. 2014;371:424–33.
Sternberg CN, Fizazi K, Saad F, Shore ND, De Giorgi U, Penson DF, et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N. Engl J Med. 2020;382:2197–206.
Safety and Efficacy Study of Enzalutamide Plus Leuprolide in Patients With Nonmetastatic Prostate Cancer (EMBARK) [Available from: https://ClinicalTrials.gov/show/NCT02319837.
Enzalutamide in Androgen Deprivation Therapy With Radiation Therapy for High Risk, Clinically Localised, Prostate Cancer [Available from: https://ClinicalTrials.gov/show/NCT02446444.
Gardner JR, Livingston PM, Fraser SF. Effects of exercise on treatment-related adverse effects for patients with prostate cancer receiving androgen-deprivation therapy: A systematic review. J Clin Oncol. 2014;32:335–46.
Chen Z, Zhang Y, Lu C, Zeng H, Schumann M, Cheng S. Supervised physical training enhances muscle strength but not muscle mass in prostate cancer patients undergoing androgen deprivation therapy: a systematic review and meta-analysis. Front Physiol. 2019;10:843.
Cormie P, Galvao DA, Spry N, Joseph D, Chee R, Taaffe DR, et al. Can supervised exercise prevent treatment toxicity in patients with prostate cancer initiating androgen-deprivation therapy: A randomised controlled trial. BJU Int. 2015;115:256–66.
Ndjavera W, Orange ST, O’Doherty AF, Leicht AS, Rochester M, Mills R, et al. Exercise-induced attenuation of treatment side-effects in patients with newly diagnosed prostate cancer beginning androgen-deprivation therapy: A randomised controlled trial. BJU Int. 2020;125:28–37.
Taaffe DR, Galvao DA, Spry N, Joseph D, Chambers SK, Gardiner RA, et al. Immediate versus delayed exercise in men initiating androgen deprivation: effects on bone density and soft tissue composition. BJU Int. 2019;123:261–9.
Newton RU, Galvao DA, Spry N, Joseph D, Chambers SK, Gardiner RA, et al. Timing of exercise for muscle strength and physical function in men initiating ADT for prostate cancer. Prostate Cancer Prostatic Dis. 2020;23:457–64.
Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, et al. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation. 2013;128:873–934.
Scott JM, Thomas SM, Peppercorn JM, Herndon JE 2nd, Douglas PS, Khouri MG, et al. Effects of exercise therapy dosing schedule on impaired cardiorespiratory fitness in patients with primary breast cancer: A randomized controlled trial. Circulation 2020;141:560–70.
Scott JM, Thomas SM, Herndon JE 2nd, Douglas PS, Yu AF, Rusch V, et al. Effects and tolerability of exercise therapy modality on cardiorespiratory fitness in lung cancer: a randomized controlled trial. J Cachexia Sarcopenia Muscle. 2021;12:1456–65.
Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, et al. Clinician’s Guide to cardiopulmonary exercise testing in adults: A scientific statement from the American Heart Association. Circulation 2010;122:191–225.
Ross RM. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:1451.
Brooks D, Solway S, Gibbons WJ. ATS statement on six-minute walk test. Am J Respir Crit Care Med. 2003;167:1287.
Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–8.
Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94.
Cella D, Nichol MB, Eton D, Nelson JB, Mulani P. Estimating clinically meaningful changes for the functional assessment of cancer therapy-prostate: Results from a clinical trial of patients with metastatic hormone-refractory prostate cancer. Value Health 2009;12:124–9.
Victorson DE, Beaumont JL, Rosenbloom SK, Shevrin D, Cella D. Efficient assessment of the most important symptoms in advanced prostate cancer: The NCCN/FACT-P Symptom Index. Psychooncology 2011;20:977–83.
Cella D, Lai JS, Chang CH, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer 2002;94:528–38.
Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10:141–6.
Kavanagh T, Mertens DJ, Hamm LF, Beyene J, Kennedy J, Corey P, et al. Prediction of long-term prognosis in 12 169 men referred for cardiac rehabilitation. Circulation 2002;106:666–71.
Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N. Engl J Med. 2002;346:793–801.
Jones LW, Watson D, Herndon JE 2nd, Eves ND, Haithcock BE, Loewen G, et al. Peak oxygen consumption and long-term all-cause mortality in nonsmall cell lung cancer. Cancer 2010;116:4825–32.
Jones LW, Courneya KS, Mackey JR, Muss HB, Pituskin EN, Scott JM, et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol. 2012;30:2530–7.
Groarke JD, Payne DL, Claggett B, Mehra MR, Gong J, Caron J, et al. Association of post-diagnosis cardiorespiratory fitness with cause-specific mortality in cancer. Eur Heart J Qual Care Clin Outcomes. 2020;6:315–22.
Segal RJ, Reid RD, Courneya KS, Sigal RJ, Kenny GP, Prud’Homme DG, et al. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J Clin Oncol. 2009;27:344–51.
Jones LW, Hornsby WE, Freedland SJ, Lane A, West MJ, Moul JW, et al. Effects of nonlinear aerobic training on erectile dysfunction and cardiovascular function following radical prostatectomy for clinically localized prostate cancer. Eur Urol. 2014;65:852–5.
Kaminsky LA, Arena R, Myers J. Reference standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing: Data from the fitness registry and the importance of exercise national database. Mayo Clin Proc. 2015;90:1515–23.
Cella D, Eton DT, Lai JS, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manag. 2002;24:547–61.
Scott JM, Zabor EC, Schwitzer E, Koelwyn GJ, Adams SC, Nilsen TS, et al. Efficacy of exercise therapy on cardiorespiratory fitness in patients with cancer: A systematic review and meta-analysis. J Clin Oncol. 2018;36:2297–305.
Alibhai SM, Breunis H, Timilshina N, Johnston C, Tomlinson G, Tannock I, et al. Impact of androgen-deprivation therapy on physical function and quality of life in men with nonmetastatic prostate cancer. J Clin Oncol. 2010;28:5038–45.
Smith MR, Saad F, Egerdie B, Sieber PR, Tammela TL, Ke C, et al. Sarcopenia during androgen-deprivation therapy for prostate cancer. J Clin Oncol. 2012;30:3271–6.
Spry NA, Taaffe DR, England PJ, Judge JS, Stephens DA, Peddle-McIntyre C, et al. Long-term effects of intermittent androgen suppression therapy on lean and fat mass: a 33-month prospective study. Prostate Cancer Prostatic Dis. 2013;16:67–72.
Sasso JP, Eves ND, Christensen JF, Koelwyn GJ, Scott J, Jones LW. A framework for prescription in exercise-oncology research. J Cachexia Sarcopenia Muscle. 2015;6:115–24.
A Study of Apalutamide in Participants With High-Risk, Localized or Locally Advanced Prostate Cancer Who Are Candidates for Radical Prostatectomy [Available from: https://ClinicalTrials.gov/show/NCT03767244.
Apalutamide With Radiotherapy and Androgen Deprivation Therapy in Prostate Cancer [Available from: https://ClinicalTrials.gov/show/NCT03488810.
Darolutamide Augments Standard Therapy for Localised Very High-Risk Cancer of the Prostate [Available from: https://ClinicalTrials.gov/show/NCT04136353.
Alibhai SMH, Santa Mina D, Ritvo P, Tomlinson G, Sabiston C, Krahn M, et al. A phase II randomized controlled trial of three exercise delivery methods in men with prostate cancer on androgen deprivation therapy. BMC Cancer. 2019;19:2.
Acknowledgements
We would like to thank all of the exercise physiologists and research staff at Duke, UNCG, and MSKCC, and especially the patients and their families, for making this study possible. M. Harrison was supported by Duke Cancer Institute (DCI) P30 CA014236 grant and the DCI shared resources for biostatistics. The work was partially funded by Department of Defense grants W81XWH-13-PCRP-CCA, W81XWH-17-2-0021 and W81XWH-14-2-0179 (MRH, AJA, DJG; Duke).
Funding
Funding for the conduct of the trial and study drug (enzalutamide) were provided by Pfizer (formerly Medivation) and Astellas through an investigator-initiated research grant to Duke University.
Author information
Authors and Affiliations
Contributions
The authors listed have made substantial contributions to the intellectual content of the paper in the various sections described as follows: conception and design (MRH, LWJ, DJG); acquisition of data (MRH, PGD, MGK, RTG, AJA, MAM, TZ, MA, KO, SE, DC, AC, BC, DJG); analysis and interpretation of data (MRH, DBB, YW, TO, DJG); drafting of the manuscript (MRH); critical revision of the manuscript for important intellectual content (MRH, PGD, MGK, DBB, AJA, MAM, TZ, MA, KO, BC, AC, LWJ, DJG); statistical analysis (YW, TO); obtaining funding (MRH, LWJ, DJG); administrative, technical or material support (MA, MM, LJW), and supervision (MRH, DJG).
Corresponding author
Ethics declarations
Competing interests
MRH has received funding from the following: Astellas (PI), AstraZeneca (PI, Consultant), Bayer (PI), Bristol Myers Squibb (PI, Consultant), Clovis Oncology (PI), Exelixis (Speaker, PI, Advisory Board, Consultant), Fujifilm (Consultant), Genentech (PI), Merck (PI), Pfizer (PI, Advisory Board, Consultant), Seattle Genetics (PI, Advisory Board, Consultant). AJA has received funding from the following: - Dendreon Valeant Corp (PI, Consultant, Other), Astellas Pharma (PI, Consultant, Other), Clovis (Advisory Board, Consultant), AstraZeneca (PI, Advisory Board, Consultant), Janssen (PI, Consultant, Other), Bayer (PI, Consultant, Other), Merck (PI, Advisory Board, Consultant), BMS (PI, Advisory Board, Consultant), Amgen (PI), Pfizer (PI, Advisory Board, Consultant), Genentech/Roche (PI), Constellation (PI), Forma (PI, Consultant). TZ has received funding from the following: Novartis (PI), Merrimack (PI), AbbVie/Stemcentrx (PI), Merck (PI), Regeneron (PI), Mirati Therapeutics (PI), Exelixis (Advisory Board), Janssen (PI, Advisory Board), Pfizer (PI, Advisory Board, Consultant), QED Therapeutics (Advisory Board), BMS (Advisory Board), Dendreon (Advisory Board), OmniSeq (PI), Personal Genome Diagnostics (PI), Aravive (Consultant), Seattle Genetics (Advisory Board), Eisai (Advisory Board), MJH Associates (Consultant), Pacific Genuity (Consultant). DJG has received funding from the following: American Association for Cancer Research (Other), Astellas (PI, Advisory Board, Consultant), AstraZeneca (PI, Advisory Board, Consultant), Axess Oncology (Other), Bayer Pharmaceuticals (Speaker, Consultant, Other), BMS (Consultant, Research, Other - Steering Committee), Calithera (Research), Capio Biosciences (Advisory Board), Constellation Pharmaceuticals (Consultant), EMD Serono (Other - Honorarium), Exelixis, Inc (Research, Consultant, Speaker, Other - Honorarium, Travel Accommodations), Flatiron (Consultant), IDEO Oncology (Consultant), Ipsen (Other - Honorarium), Janssen Pharmaceuticals (Research, Consultant, Other - Independent Data Monitoring Committee), Merck Sharp & Dohme (Consultant), Michael J Hennessey Associates (Consultant, Other - Honorarium), Millennium Medical Publishing (Other - Co-Editor-in-Chief), Modra Pharmaceuticals B.V. (Advisory Board), Myovant Sciences, Inc (Consultant), NCI Genitourinary Steering Committee (Other - Member), Nektar Therapeutics (Other - Steering Committee), Novartis (Research), Physician Education Resource, LLC (Consultant), Pfizer (Research, Consultant, Other - Steering Committee, Honorarium), Propella TX (Consultant), RevHealth, LLC (Consultant), Sanofi (Research, Consultant, Speaker, Other - Honorarium, Travel Accommodations), UroGPO (Other - Honorarium), UroToday (Other - Honorarium, Travel Accommodations), Xcures (Consultant). PGD, MGK, DBB, RTG, MAM, MA, KO, SE, DC, MM, YW, TO, BC, AC, and LWJ declare no competing financial interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Harrison, M.R., Davis, P.G., Khouri, M.G. et al. A randomized controlled trial comparing changes in fitness with or without supervised exercise in patients initiated on enzalutamide and androgen deprivation therapy for non-metastatic castration-sensitive prostate cancer (EXTEND). Prostate Cancer Prostatic Dis 25, 58–64 (2022). https://doi.org/10.1038/s41391-022-00519-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-022-00519-4
This article is cited by
-
Working hard or hardly working? A brief commentary of latest research on exercise and prostate cancer
Prostate Cancer and Prostatic Diseases (2023)
-
Cardiovascular adverse events-related to GnRH agonists and GnRH antagonists: analysis of real-life data from Eudra-Vigilance and Food and Drug Administration databases entries
Prostate Cancer and Prostatic Diseases (2023)