Abstract
Background
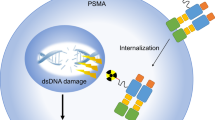
Targeted radionuclide therapy with Actinium-225-labeled prostate-specific membrane antigen ligands (225Ac-PSMA) has emerged as a promising treatment modality in the management of metastatic castration-resistant prostate cancer (mCRPC). With its high linear energy transfer and short path length, 225Ac induces double-stranded DNA breaks and is expected to have excellent efficacy and safety profile. This systematic review was conducted to precisely evaluate the role of 225Ac-PSMA radioligand therapy (RLT) in mCRPC.
Methods
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. Searches were made using relevant keywords in the PubMed, Embase, and Scopus databases, and articles up to December 2020 were included. Data on efficacy and toxicity were extracted from the individual articles. Random-effects model was used for generating pooled estimates through meta-analysis.
Results
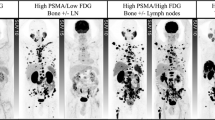
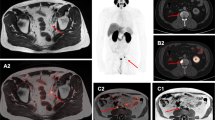
Ten articles comprising 256 patients were included. Overall, 62.8% (95% confidence interval, CI: 53.4–71.7%) of the patients treated with 225Ac-PSMA RLT achieved biochemical response, i.e., ≥50% decline in the serum prostate-specific antigen levels from baseline. Molecular response on Gallium-68 PSMA positron emission tomography/computed tomography was noted in 74% (95% CI: 50.1–92.1%) of the patients. The pooled estimates of median progression-free survival and overall survival were 9.1 months (95% CI: 3.6–14.5 months) and 12.8 months (95% CI: 4.5–21.0 months), respectively. The most commonly reported adverse event was xerostomia, which was observed in 72.7% (95% CI: 50.5–90.1%) of the patients. However, clinically significant toxicity was limited with grade ≥3 xerostomia, anemia, leucopenia, thrombocytopenia, and nephrotoxicity occurring in 1.2%, 12.3%, 8.3%, 6.3%, and 3.8% of the patients, respectively. Treatment discontinuation due to adverse events was noted in 20/208 patients.
Conclusions
225Ac-PSMA RLT is an efficacious and safe treatment option for patients with mCRPC. Future randomized controlled trials are required to establish its therapeutic efficacy and survival benefit vis-à-vis other approved treatment modalities.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Helgstrand JT, Røder MA, Klemann N, Toft BG, Lichtensztajn DY, Brooks JD, et al. Trends in incidence and 5‐year mortality in men with newly diagnosed, metastatic prostate cancer—a population‐based analysis of 2 national cohorts. Cancer. 2018;124:2931–8.
Ingrosso G, Detti B, Scartoni D, Lancia A, Giacomelli I, Baki M, et al. Current therapeutic options in metastatic castration-resistant prostate cancer. Semin Oncol. 2018;45:303–15.
Wright GL Jr, Haley C, Beckett ML, Schellhammer PF. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol Oncol. 1995;1:18–28.
Yadav MP, Ballal S, Sahoo RK, Dwivedi SN, Bal C. Radioligand therapy with 177Lu-PSMA for metastatic castration-resistant prostate cancer: a systematic review and meta-analysis. AJR Am J Roentgenol. 2019;213:275–85.
Kratochwil C, Giesel FL, Heussel CP, Kazdal D, Endris V, Nientiedt C, et al. Patients resistant against PSMA-targeting α-radiation therapy often harbor mutations in DNA damage-repair-associated genes. J Nucl Med. 2020;61:683–8.
Tafreshi NK, Doligalski ML, Tichacek CJ, Pandya DN, Budzevich MM, El-Haddad G, et al. Development of targeted alpha particle therapy for solid tumors. Molecules. 2019;24:4314.
Kratochwil C, Bruchertseifer F, Giesel FL, Weis M, Verburg FA, Mottaghy F, et al. 225Ac-PSMA-617 for PSMA-targeted α-radiation therapy of metastatic castration-resistant prostate cancer. J Nucl Med. 2016;57:1941–4.
De Vincentis G, Gerritsen W, Gschwend JE, Hacker M, Lewington V, O’Sullivan JM, et al. Advances in targeted alpha therapy for prostate cancer. Ann Oncol. 2019;30:1728–39.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016;34:1402–18.
Seitz AK, Rauscher I, Haller B, Krönke M, Luther S, Heck MM, et al. Preliminary results on response assessment using 68Ga-HBED-CC-PSMA PET/CT in patients with metastatic prostate cancer undergoing docetaxel chemotherapy. Eur J Nucl Med Mol Imaging. 2018;45:602–12.
Schmitz S, Maguire Á, Morris J, Ruggeri K, Haller E, Kuhn I, et al. The use of single armed observational data to closing the gap in otherwise disconnected evidence networks: a network meta-analysis in multiple myeloma. BMC Med Res Methodol. 2018;18:66.
Kratochwil C, Bruchertseifer F, Rathke H, Bronzel M, Apostolidis C, Weichert W, et al. Targeted α-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: dosimetry estimate and empiric dose finding. J Nucl Med. 2017;58:1624–31.
Kratochwil C, Bruchertseifer F, Rathke H, Hohenfellner M, Giesel FL, Haberkorn U, et al. Targeted α-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: swimmer-plot analysis suggests efficacy regarding duration of tumor control. J Nucl Med. 2018;59:795–802.
Sathekge M, Bruchertseifer F, Knoesen O, Reyneke F, Lawal I, Lengana T, et al. 225Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: a pilot study. Eur J Nucl Med Mol Imaging. 2019;46:129–38.
Sathekge M, Bruchertseifer F, Vorster M, Lawal IO, Knoesen O, Mahapane J, et al. Predictors of overall and disease-free survival in metastatic castration-resistant prostate cancer patients receiving 225Ac-PSMA-617 radioligand therapy. J Nucl Med. 2020;61:62–9.
Khreish F, Ebert N, Ries M, Maus S, Rosar F, Bohnenberger H, et al. 225Ac-PSMA-617/177Lu-PSMA-617 tandem therapy of metastatic castration-resistant prostate cancer: pilot experience. Eur J Nucl Med Mol Imaging. 2020;47:721–8.
Yadav MP, Ballal S, Sahoo RK, Tripathi M, Seth A, Bal C. Efficacy and safety of 225Ac-PSMA-617 targeted alpha therapy in metastatic castration-resistant Prostate Cancer patients. Theranostics. 2020;10:9364–77.
Zacherl MJ, Gildehaus FJ, Mittlmeier L, Boening G, Gosewisch A, Wenter V, et al. First clinical results for PSMA targeted alpha therapy using 225Ac-PSMA-I&T in advanced mCRPC patients. J Nucl Med. 2020. https://doi.org/10.2967/jnumed.120.251017.
Satapathy S, Mittal BR, Sood A, Das CK, Singh SK, Mavuduru RS, et al. Health-related quality-of-life outcomes with actinium-225-prostate specific membrane antigen-617 therapy in patients with heavily pretreated metastatic castration-resistant prostate cancer. Indian J Nucl Med. 2020;35:299–304.
Feuerecker B, Tauber R, Knorr K, Heck M, Beheshti A, Seidl C, et al. Activity and adverse events of actinium-225-PSMA-617 in advanced metastatic castration-resistant prostate cancer after failure of lutetium-177-PSMA. Eur Urol. 2021;79:343–50.
van der Doelen MJ, Mehra N, van Oort IM, Looijen-Salamon MG, Janssen MJR, Custers JAE, et al. Clinical outcomes and molecular profiling of advanced metastatic castration-resistant prostate cancer patients treated with 225Ac-PSMA-617 targeted alpha-radiation therapy. Urol Oncol. 2020. https://doi.org/10.1016/j.urolonc.2020.12.002.
Barber TW, Singh A, Kulkarni HR, Niepsch K, Billah B, Baum RP. Clinical outcomes of 177Lu-PSMA radioligand therapy in earlier and later phases of metastatic castration-resistant prostate cancer grouped by previous taxane chemotherapy. J Nucl Med. 2019;60:955–62.
Kessel K, Seifert R, Schäfers M, Weckesser M, Schlack K, Boegemann M, et al. Second line chemotherapy and visceral metastases are associated with poor survival in patients with mCRPC receiving 177Lu-PSMA-617. Theranostics. 2019;9:4841–8.
Satapathy S, Mittal BR, Sood A. Visceral metastases as predictors of response and survival outcomes in patients of castration-resistant prostate cancer treated With 177Lu-labeled prostate-specific membrane antigen radioligand therapy: a systematic review and meta-analysis. Clin Nucl Med. 2020;45:935–42.
Suman S, Parghane RV, Joshi A, Prabhash K, Bakshi G, Talole S, et al. Therapeutic efficacy, prognostic variables and clinical outcome of 177Lu-PSMA-617 PRLT in progressive mCRPC following multiple lines of treatment: prognostic implications of high FDG uptake on dual tracer PET-CT vis-à-vis Gleason score in such cohort. Br J Radio. 2019;92:20190380.
Mínguez Gabiña P, Roeske JC, Mínguez R, Rodeño E, Gómez de Iturriaga A. Microdosimetry-based determination of tumor control probability curves for treatments with 225Ac-PSMA of metastatic castration resistant prostate cancer. Phys Med Biol. 2020;65:235012.
Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825–33.
Author information
Authors and Affiliations
Contributions
SS was responsible for designing the review protocol, conducting the search, screening potentially eligible studies, extracting and analyzing data, and writing the manuscript. AS was responsible for conducting the search, screening potentially eligible studies, extracting and analyzing data, interpreting results, and revising the manuscript. CKD and BRM critically reviewed the manuscript and provided feedback on the same.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Satapathy, S., Sood, A., Das, C.K. et al. Evolving role of 225Ac-PSMA radioligand therapy in metastatic castration-resistant prostate cancer—a systematic review and meta-analysis. Prostate Cancer Prostatic Dis 24, 880–890 (2021). https://doi.org/10.1038/s41391-021-00349-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-021-00349-w
This article is cited by
-
Implementing Ac-225 labelled radiopharmaceuticals: practical considerations and (pre-)clinical perspectives
EJNMMI Radiopharmacy and Chemistry (2024)
-
Highlight selection of radiochemistry and radiopharmacy developments by editorial board
EJNMMI Radiopharmacy and Chemistry (2023)
-
Radiotheranostics in advanced prostate cancer: Current and future directions
Prostate Cancer and Prostatic Diseases (2023)
-
Editor’ summary: A paradigm shift in castration-resistant prostate cancer management
Prostate Cancer and Prostatic Diseases (2022)
-
Molecular Imaging, How Close to Clinical Precision Medicine in Lung, Brain, Prostate and Breast Cancers
Molecular Imaging and Biology (2022)