Abstract
Background
Monotherapy with immune checkpoint inhibitors has generally been unsuccessful in men with advanced prostate cancer. Preclinical data support the notion that cryotherapy may improve immune-mediated and anti-tumor responses. The objective of this study was to assess the safety and feasibility of whole-prostate gland cryotherapy combined with pembrolizumab and androgen deprivation in men with oligometastatic hormone-sensitive prostate cancer.
Methods
This single-institution, pilot trial recruited 12 patients with newly diagnosed oligometastatic prostate cancer between 2015 and 2016. Patients underwent whole-prostate cryoablation combined with short-term androgen deprivation (eight months) and pembrolizumab (6 doses). The primary clinical endpoints were the number of patients with a PSA level of <0.6 ng/mL at one year and the frequency of adverse events. Other outcome measures included progression-free survival and systemic therapy-free survival. Exploratory analyses included PD-L1 protein expression.
Results
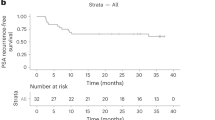
Forty two percent (5/12) of patients had a PSAs of <0.6 ng/mL at one year though only 2 of these patients had recovered their testosterone at this time point. Median progression-free survival was 14 months, and median systemic therapy-free survival was 17.5 months. PD-L1 expression was not detectable by IHC in patients with evaluable tissue. All adverse events were grade ≤2, and there were no apparent complications from cryotherapy.
Conclusions
Whole-prostate cryoablation combined with short-term androgen deprivation and pembrolizumab treatment was well tolerated and no safety concerns were observed in men with oligometastatic prostate cancer. Though local disease appeared effectively treated in the majority of men, the regimen only infrequency led to sustained disease control following testosterone recovery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–17.
Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–92.
Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumus M, Mazieres J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379:2040–51.
Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378:2093–104.
Topalian SL. Targeting immune checkpoints in cancer therapy. JAMA. 2017;318:1647–8.
Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–68.
Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–8.
Wu YM, Cieslik M, Lonigro RJ, Vats P, Reimers MA, Cao X, et al. Inactivation of CDK12 delineates a distinct immunogenic class of advanced prostate cancer. Cell. 2018;173:1770–82. e1714.
Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–13.
Antonarakis ES. Cyclin-dependent kinase 12, immunity, and prostate cancer. N Engl J Med. 2018;379:1087–9.
Abida W, Cheng ML, Armenia J, Middha S, Autio KA, Vargas HA, et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol. 2019;5:471–478. [Epub ahead of print]
Isaacsson Velho P, Antonarakis ES. PD-1/PD-L1 pathway inhibitors in advanced prostate cancer. Expert Rev Clin Pharm. 2018;11:475–86.
Comiskey MC, Dallos MC, Drake CG. Immunotherapy in prostate cancer: teaching an old dog new tricks. Curr Oncol Rep. 2018;20:75.
Abdo J, Cornell DL, Mittal SK, Agrawal DK. Immunotherapy plus cryotherapy: potential augmented abscopal effect for advanced cancers. Front Oncol. 2018;8:85.
Benzon B, Glavaris SA, Simons BW, Hughes RM, Ghabili K, Mullane P, et al. Combining immune check-point blockade and cryoablation in an immunocompetent hormone sensitive murine model of prostate cancer. Prostate Cancer Prostatic Dis. 2018;21:126–36.
Levy DA, Pisters LL, Jones JS. Prognostic value of initial prostate-specific antigen levels after salvage cryoablation for prostate cancer. BJU Int. 2010;106:986–90.
Levy DA, Ross AE, ElShafei A, Krishnan N, Hatem A, Jones JS. Definition of biochemical success following primary whole gland prostate cryoablation. J Urol. 2014;192:1380–4.
Mercader M, Bodner BK, Moser MT, Kwon PS, Park ES, Manecke RG, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci USA. 2001;98:14565–70.
Gannon PO, Poisson AO, Delvoye N, Lapointe R, Mes-Masson AM, Saad F. Characterization of the intra-prostatic immune cell infiltration in androgen-deprived prostate cancer patients. J Immunol Methods. 2009;348:9–17.
Mercader M, Sengupta S, Bodner BK, Manecke RG, Cosar EF, Moser MT, et al. Early effects of pharmacological androgen deprivation in human prostate cancer. BJU Int. 2007;99:60–7.
Nam W, Choi SY, Yoo SJ, Ryu J, Lee J, Kyung YS, et al. Factors associated with testosterone recovery after androgen deprivation therapy in patients with prostate cancer. Investig Clin Urol. 2018;59:18–24.
Crook JM, O’Callaghan CJ, Duncan G, Dearnaley DP, Higano CS, Horwitz EM, et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med. 2012;367:895–903.
Murthy V, Norman AR, Shahidi M, Parker CC, Horwich A, Huddart RA, et al. Recovery of serum testosterone after neoadjuvant androgen deprivation therapy and radical radiotherapy in localized prostate cancer. BJU Int. 2006;97:476–9.
Ahmed KA, Barney BM, Davis BJ, Park SS, Kwon ED, Olivier KR. Stereotactic body radiation therapy in the treatment of oligometastatic prostate cancer. Front Oncol. 2012;2:215.
Cha E, Klinger M, Hou Y, Cummings C, Ribas A, Faham M, et al. Improved survival with T cell clonotype stability after anti-CTLA-4 treatment in cancer patients. Sci Transl Med. 2014;6:238ra270.
Cotliar J, Querfeld C, Boswell WJ, Raja N, Raz D, Chen R. Pembrolizumab-associated sarcoidosis. JAAD Case Rep. 2016;2:290–3.
Schellhammer PF, Chodak G, Whitmore JB, Sims R, Frohlich MW, Kantoff PW. Lower baseline prostate-specific antigen is associated with a greater overall survival benefit from sipuleucel-T in the Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) trial. Urology. 2013;81:1297–302.
Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700–12.
Beer TM, Kwon ED, Drake CG, Fizazi K, Logothetis C, Gravis G, et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol. 2017;35:40–7.
Shen YC, Ghasemzadeh A, Kochel CM, Nirschl TR, Francica BJ, Lopez-Bujanda ZA, et al. Combining intratumoral Treg depletion with androgen deprivation therapy (ADT): preclinical activity in the Myc-CaP model. Prostate Cancer Prostatic Dis. 2018;21:113–25.
Drake CG, Doody AD, Mihalyo MA, Huang CT, Kelleher E, Ravi S, et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7:239–49.
Parker CC, James ND, Brawley CD, Clarke NW, Hoyle AP, Ali A, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392:2353–66.
Boeve LMS, Hulshof M, Vis AN, Zwinderman AH, Twisk JWR, Witjes WPJ, et al. Effect on survival of androgen deprivation therapy alone compared to androgen deprivation therapy combined with concurrent radiation therapy to the prostate in patients with primary bone metastatic prostate cancer in a prospective randomised clinical trial: data from the HORRAD trial. Eur Urol. 2019;75:410–8.
Sheng MX, Wan LL, Liu CM, Liu CX, Chen SS. Cytoreductive cryosurgery in patients with bone metastatic prostate cancer: a retrospective analysis. Kaohsiung J Med Sci. 2017;33:609–15.
Chi KN, Agarwal N, Bjartell A, Chung BH, Pereira de Santana Gomes AJ, Given R, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381:13–24.
Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381:121–31.
James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338–51.
Acknowledgements
We are grateful to the patients and their families for participating in this trial. This work was supported by PCF young investigator award (Ashley E. Ross) as well as partial funding from Merck and Healthtronics. Paula J. Hurley acknowledges support from the American Cancer Society (131356-RSG-17-160-01-CSM) and The National Cancer Institute/National Institute of Health RO1CA211695-01A1. P.T.T. acknowledges support from Ronald Rose, Joan Lazar, Movember Foundation, Prostate Cancer Foundation; NIH/NCI (R01CA166348, U01CA212007, U01CA231776 and R21CA223403). E.S.A. is partially funded by National Institutes of Health Cancer Center Support Grant P30 CA006973, and by Department of Defense grant W81XWH-16-PCRP-CCRSA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
AER has previously been a consultant for Healthtronics. PTT has grant support from Astellas Pharm., RefleXion Medical, Inc and Bayer Healthcare; and has consulted for RefleXion Medical, Inc. CGD acknowledges stock or ownership interests in Compugen, Harpoon, Kleo, Potenza, and Tizona Therapeutics, and has served as a consultant for Agenus, Dendreon, Janssen Oncology, Eli Lilly, Merck, AstraZeneca, MedImmune, Pierre Fabre, Genentech, and Genocea Biosciences. ESA is a paid consultant/advisor to Janssen, Astellas, Sanofi, Dendreon, Medivation, AstraZeneca, Clovis, and Merck; he has received research funding to his institution from Janssen, Johnson & Johnson, Sanofi, Dendreon, Genentech, Novartis, Tokai, Bristol Myers-Squibb, AstraZeneca, Clovis, and Merck; and he is the co-inventor of an AR-V7 biomarker technology that has been licensed to Qiagen. The other authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Ross, A.E., Hurley, P.J., Tran, P.T. et al. A pilot trial of pembrolizumab plus prostatic cryotherapy for men with newly diagnosed oligometastatic hormone-sensitive prostate cancer. Prostate Cancer Prostatic Dis 23, 184–193 (2020). https://doi.org/10.1038/s41391-019-0176-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-019-0176-8
This article is cited by
-
Intratumoural immunotherapy plus focal thermal ablation for localized prostate cancer
Nature Reviews Urology (2024)
-
Local Therapeutics for the Treatment of Oligo Metastatic Prostate Cancer
Current Urology Reports (2023)
-
Recent advances in the molecular targeted drugs for prostate cancer
International Urology and Nephrology (2023)
-
Advances in bio-immunotherapy for castration-resistant prostate cancer
Journal of Cancer Research and Clinical Oncology (2023)
-
Focal therapy for primary tumor and metastases in de novo or recurrent oligometastatic prostate cancer: current standing and future perspectives
World Journal of Urology (2022)