Abstract
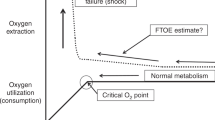
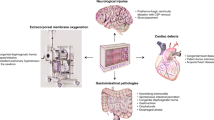
In this narrative review, we summarize the current knowledge and applications of somatic near-infrared spectroscopy (NIRS), with a focus on intestinal, renal, limb, and multi-site applications in neonates. Assessing somatic oxygenation at various body locations in neonates may aid in the understanding of underlying pathophysiology of organ injury. Considering cerebral autoregulation may be active to protect the brain during systemic circulatory failure, peripheral somatic oxygenation may potentially provide an early indication of neonatal cardiovascular failure and ultimate hypoxemic injury to vital organs including the brain. Certain intestinal oxygenation patterns appear to be associated with the onset and course of necrotizing enterocolitis, whereas impaired renal oxygenation may indicate the onset of acute kidney injury after various types of hypoxic events. Peripheral muscle oxygenation measured at a limb may be particularly effective in the early prediction of shock in neonates. Using multi-site NIRS may complement current approaches and clinical investigations to alert for neonatal tissue hypoxemia, and potentially even guide management. However, somatic NIRS has its inherent limitations in regard to accuracy. Interpretation of organ-specific values can also be challenging. Last, currently there are limited prospective intervention studies, and clinical benefits need to be examined further, after the clarification of critical threshold-values.
Impact
-
The assessment of somatic oxygenation using NIRS may contribute to the prediction of specific diseases in hemodynamically challenged neonates. Furthermore, it may give early warning signs for impending cardiovascular failure, and impaired cerebral circulation and oxygenation.
-
We present a comprehensive overview of the literature on applications of NIRS to various somatic areas, with a focus on its potential clinical applicability, including future research directions.
-
This paper will enable prospective standardized studies, and multicenter collaboration to obtain statistical power, likely to advance the field.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Yoxall, C. W. & Weindling, A. M. The measurement of peripheral venous oxyhemoglobin saturation in newborn infants by near infrared spectroscopy with venous occlusion. Pediatr. Res. 39, 1103–1106 (1996).
Wardle, S. P. & Weindling, A. M. Peripheral oxygenation in preterm infants. Clin. Perinatol. 26, 947–966, ix–x (1999).
Brazy, J. E., Lewis, D. V., Mitnick, M. H. & Jobsis vander Vliet, F. F. Noninvasive monitoring of cerebral oxygenation in preterm infants: preliminary observations. Pediatrics 75, 217–225 (1985).
Wyatt, J. S., Cope, M., Delpy, D. T., Wray, S. & Reynolds, E. O. Quantification of cerebral oxygenation and haemodynamics in sick newborn infants by near infrared spectrophotometry. Lancet 2, 1063–1066 (1986).
Fujisaka, S. et al. A clinical tissue oximeter using NIR time-resolved spectroscopy. Adv. Exp. Med. Biol. 876, 427–433 (2016).
Matcher, S. J. & Cooper, C. E. Absolute quantification of deoxyhaemoglobin concentration in tissue near infrared spectroscopy. Phys. Med. Biol. 39, 1295–1312 (1994).
Buckley, N. M., Brazeau, P. & Frasier, I. D. Intestinal and femoral blood flow autoregulation in developing swine. Biol. Neonate 49, 229–240 (1986).
Dotinga, B. M. et al. Maturation of intestinal oxygenation: a review of mechanisms and clinical implications for preterm neonates. Front. Pediatr. 8, 354 (2020).
Greisen, G. Cerebral blood flow and energy metabolism in the newborn. Clin. Perinatol. 24, 531–546 (1997).
Tan, C. O., Hamner, J. W. & Taylor, J. A. The role of myogenic mechanisms in human cerebrovascular regulation. J. Physiol. 591, 5095–5105 (2013).
Luria, O. et al. The role of blood flow distribution in the regulation of cerebral oxygen availability in fetal growth restriction. Med. Eng. Phys. 34, 364–369 (2012).
Hampl, V. & Jakoubek, V. Regulation of fetoplacental vascular bed by hypoxia. Physiol. Res. 58, S87–S93 (2009).
Tweed, W. A., Cote, J., Wade, J. G., Gregory, G. & Mills, A. Preservation of fetal brain blood flow relative to other organs during hypovolemic hypotension. Pediatr. Res. 16, 137–140 (1982).
Mittnacht, A. J. Near infrared spectroscopy in children at high risk of low perfusion. Curr. Opin. Anaesthesiol. 23, 342–347 (2010).
Regan, M. C., Young, L. S., Geraghty, J. & Fitzpatrick, J. M. Regional renal blood flow in normal and disease states. Urol. Res. 23, 1–10 (1995).
Granger, D. N., Holm, L. & Kvietys, P. The gastrointestinal circulation: physiology and pathophysiology. Compr. Physiol. 5, 1541–1583 (2015).
Kooi, E. M. W. & Richter, A. E. Cerebral autoregulation in sick infants: current insights. Clin. Perinatol. 47, 449–467 (2020).
Kaufman, J., Almodovar, M. C., Zuk, J. & Friesen, R. H. Correlation of abdominal site near-infrared spectroscopy with gastric tonometry in infants following surgery for congenital heart disease. Pediatr. Crit. Care Med. 9, 62–68 (2008).
Hoffman, G. M. et al. Postoperative cerebral and somatic near-infrared spectroscopy saturations and outcome in hypoplastic left heart syndrome. Ann. Thorac. Surg. 103, 1527–1535 (2017).
Corvaglia, L. et al. Splanchnic oxygenation at first enteral feeding in preterm infants: correlation with feeding intolerance. J. Pediatr. Gastroenterol. Nutr. 64, 550–554 (2017).
Schat, T. E. et al. Early cerebral and intestinal oxygenation in the risk assessment of necrotizing enterocolitis in preterm infants. Early Hum. Dev. 131, 75–80 (2019).
van der Heide, M., Hulscher, J. B. F., Bos, A. F. & Kooi, E. M. W. Near-infrared spectroscopy as a diagnostic tool for necrotizing enterocolitis in preterm infants. Pediatr. Res. 90, 148–155 (2020).
Palleri, E., Wackernagel, D., Wester, T. & Bartocci, M. Low splanchnic oxygenation and risk for necrotizing enterocolitis in extremely preterm newborns. J. Pediatr. Gastroenterol. Nutr. 71, 401–406 (2020).
Booth, E. A., Dukatz, C., Ausman, J. & Wider, M. Cerebral and somatic venous oximetry in adults and infants. Surg. Neurol. Int. 1, 75 (2010).
Vesoulis, Z. A., Mintzer, J. P. & Chock, V. Y. Neonatal NIRS monitoring: recommendations for data capture and review of analytics. J. Perinatol. 41, 675–688 (2021).
Bozzetti, V. et al. Impact of continuous vs bolus feeding on splanchnic perfusion in very low birth weight infants: a randomized trial. J. Pediatr. 176, 86.e82–92.e82 (2016).
Corvaglia, L. et al. Bolus vs. continuous feeding: effects on splanchnic and cerebral tissue oxygenation in healthy preterm infants. Pediatr. Res. 76, 81–85 (2014).
Dani, C. et al. Splanchnic tissue oxygenation for predicting feeding tolerance in preterm infants. JPEN J. Parenter. Enter. Nutr. 39, 935–940 (2015).
Martini, S., Aceti, A., Beghetti, I., Faldella, G. & Corvaglia, L. Feed-related splanchnic oxygenation in preterm infants with abnormal antenatal doppler developing gut complications. J. Pediatr. Gastroenterol. Nutr. 66, 755–759 (2018).
Al-Hamad, S. et al. Contrast-enhanced ultrasound and near-infrared spectroscopy of the neonatal bowel: novel, bedside, noninvasive, and radiation-free imaging for early detection of necrotizing enterocolitis. Am. J. Perinatol. 35, 1358–1365 (2018).
DeWitt, A. G., Charpie, J. R., Donohue, J. E., Yu, S. & Owens, G. E. Splanchnic near-infrared spectroscopy and risk of necrotizing enterocolitis after neonatal heart surgery. Pediatr. Cardiol. 35, 1286–1294 (2014).
Gay, A. N. et al. Near-infrared spectroscopy measurement of abdominal tissue oxygenation is a useful indicator of intestinal blood flow and necrotizing enterocolitis in premature piglets. J. Pediatr. Surg. 46, 1034–1040 (2011).
Schat, T. E. et al. The relation between splanchnic ischaemia and intestinal damage in necrotising enterocolitis. Arch. Dis. Child. Fetal Neonatal Ed. 101, F533–F538 (2016).
Banerjee, J., Leung, T. S. & Aladangady, N. Effect of blood transfusion on intestinal blood flow and oxygenation in extremely preterm infants during first week of life. Transfusion 56, 808–815 (2016).
Marin, T. et al. Red blood cell transfusion-related necrotizing enterocolitis in very-low-birthweight infants: a near-infrared spectroscopy investigation. Transfusion 53, 2650–2658 (2013).
Marin, T. & Moore, J. E. Mesenteric oxygenation changes associated with necrotizing enterocolitis and pneumoperitoneum after multiple blood transfusions: a case report. Adv. Neonatal Care 18, 121–127 (2018).
Bailey, S. M., Hendricks-Munoz, K. D. & Mally, P. V. Variability in splanchnic tissue oxygenation during preterm red blood cell transfusion given for symptomatic anaemia may reveal a potential mechanism of transfusion-related acute gut injury. Blood Transfus. 13, 429–434 (2015).
Sandal, G. et al. Assessment of red blood cell transfusion and transfusion duration on cerebral and mesenteric oxygenation using near-infrared spectroscopy in preterm infants with symptomatic anemia. Transfusion 54, 1100–1105 (2014).
White, L., Said, M. & Rais-Bahrami, K. Monitoring mesenteric tissue oxygenation with near-infrared spectroscopy during packed red blood cell transfusion in preterm infants. J. Neonatal Perinat. Med. 8, 157–163 (2015).
Kalteren, W. S. et al. The short-term effects of rbc transfusions on intestinal injury in preterm infants. Pediatr. Res. 93, 1307–1313 (2023).
Kalteren, W. S. et al. Red blood cell transfusions affect intestinal and cerebral oxygenation differently in preterm infants with and without subsequent necrotizing enterocolitis. Am. J. Perinatol. 35, 1031–1037 (2018).
Bailey, S. M. & Mally, P. V. Review of splanchnic oximetry in clinical medicine. J. Biomed. Opt. 21, 091306 (2016).
Grometto, A., Pizzo, B., Strozzi, M. C., Gazzolo, F. & Gazzolo, D. Near-infrared spectroscopy is a promising noninvasive technique for monitoring the effects of feeding regimens on the cerebral and splanchnic regions. Acta Paediatr. 107, 234–239 (2018).
Martini, S. & Corvaglia, L. Splanchnic NIRS monitoring in neonatal care: rationale, current applications and future perspectives. J. Perinatol. 38, 431–443 (2018).
Thompson, A., Benni, P., Seyhan, S. & Ehrenkranz, R. Meconium and transitional stools may cause interference with near-infrared spectroscopy measurements of intestinal oxygen saturation in preterm infants. Adv. Exp. Med. Biol. 765, 287–292 (2013).
Isler, H. et al. Absorption spectra of early stool from preterm infants need to be considered in abdominal NIRS oximetry. Biomed. Opt. express 10, 2784–2794 (2019).
Said, M. M., Niforatos, N. & Rais-Bahrami, K. Validation of near infrared spectroscopy to measure abdominal somatic tissue oxygen saturation in neonates. J. Neonatal Perinat. Med. 6, 23–30 (2013).
Mintzer, J. P., Parvez, B., Chelala, M., Alpan, G. & LaGamma, E. F. Quiescent variability of cerebral, renal, and splanchnic regional tissue oxygenation in very low birth weight neonates. J. Neonatal Perinat. Med. 7, 199–206 (2014).
Pocivalnik, M. et al. Regional tissue oxygen saturation: comparability and reproducibility of different devices. J. Biomed. Opt. 16, 057004 (2011).
Dix, L. M., van Bel, F., Baerts, W. & Lemmers, P. M. Comparing near-infrared spectroscopy devices and their sensors for monitoring regional cerebral oxygen saturation in the neonate. Pediatr. Res. 74, 557–563 (2013).
Hyttel-Sorensen, S., Sorensen, L. C., Riera, J. & Greisen, G. Tissue oximetry: a comparison of mean values of regional tissue saturation, reproducibility and dynamic range of four NIRS-instruments on the human forearm. Biomed. Opt. Express 2, 3047–3057 (2011).
Kleiser, S., Nasseri, N., Andresen, B., Greisen, G. & Wolf, M. Comparison of tissue oximeters on a liquid phantom with adjustable optical properties. Biomed. Opt. Express 7, 2973–2992 (2016).
Kalteren, W. S., Bos, A. F., van Oeveren, W., Hulscher, J. B. F. & Kooi, E. M. W. Neonatal anemia relates to intestinal injury in preterm infants. Pediatr. Res. 91, 1452–1458 (2021).
Dotinga, B. M., Solberg, R., Saugstad, O. D., Bos, A. F. & Kooi, E. M. W. Splanchnic oxygen saturation during reoxygenation with 21% or 100% O2 in newborn piglets. Pediatr. Res. 92, 445–452 (2021).
Mintzer, J. P., Parvez, B. & La Gamma, E. F. Regional tissue oxygen extraction and severity of anemia in very low birth weight neonates: a pilot NIRS analysis. Am. J. Perinatol. 35, 1411–1418 (2018).
Goldshtrom, N., Isler, J. R. & Sahni, R. Comparing liver and lower abdomen near-infrared spectroscopy in preterm infants. Early Hum. Dev. 151, 105194 (2020).
Howarth, C. N. et al. Regional cerebral and splanchnic tissue oxygen saturation in preterm infants - longitudinal normative measurements. Early Hum. Dev. 165, 105540 (2022).
Chock, V. Y. et al. Early brain and abdominal oxygenation in extremely low birth weight infants. Pediatr. Res. 92, 1034–1041 (2022).
van der Heide, M. et al. Regional splanchnic oxygen saturation for preterm infants in the first week after birth: reference values. Pediatr. Res. 90, 882–887 (2021).
Gillam-Krakauer, M. et al. Correlation of abdominal rSO2 with superior mesenteric artery velocities in preterm infants. J. Perinatol. 33, 609–612 (2013).
Akotia, D. H., Durham, J. T., Arnell, K. M., Petruzzelli, D. L. & Katheria, A. C. Relationship between near-infrared spectroscopy and transabdominal ultrasonography: noninvasive monitoring of intestinal function in neonates. Med. Sci. Monit. 22, 61–68 (2016).
Bailey, S. M., Hendricks-Munoz, K. D., Wells, J. T. & Mally, P. Packed red blood cell transfusion increases regional cerebral and splanchnic tissue oxygen saturation in anemic symptomatic preterm infants. Am. J. Perinatol. 27, 445–453 (2010).
Dani, C., Pratesi, S., Fontanelli, G., Barp, J. & Bertini, G. Blood transfusions increase cerebral, splanchnic, and renal oxygenation in anemic preterm infants. Transfusion 50, 1220–1226 (2010).
Mintzer, J. P., Parvez, B., Chelala, M., Alpan, G. & LaGamma, E. F. Monitoring regional tissue oxygen extraction in neonates <1250 g helps identify transfusion thresholds independent of hematocrit. J. Neonatal Perinat. Med. 7, 89–100 (2014).
Ledo, A., Aguar, M., Nunez-Ramiro, A., Saenz, P. & Vento, M. Abdominal near-infrared spectroscopy detects low mesenteric perfusion early in preterm infants with hemodynamic significant ductus arteriosus. Neonatology 112, 238–245 (2017).
Cortez, J. et al. Noninvasive evaluation of splanchnic tissue oxygenation using near-infrared spectroscopy in preterm neonates. J. Matern. Fetal Neonatal Med. 24, 574–582 (2011).
Mishra, V., Mathur, A. A., Mohamed, S. & Maheshwari, A. Role of near-infrared spectroscopy in the diagnosis and assessment of necrotizing enterocolitis. Newborn 1, 177–181 (2022).
Howarth, C., Banerjee, J., Eaton, S. & Aladangady, N. Biomarkers of gut injury in neonates - where are we in predicting necrotising enterocolitis? Front. Pediatr. 10, 1048322 (2022).
Howarth, C., Banerjee, J., Leung, T. & Aladangady, N. Could near infrared spectroscopy (NIRS) be the new weapon in our fight against necrotising enterocolitis? Front. Pediatr. 10, 1024566 (2022).
Patel, A. K. et al. Abdominal near-infrared spectroscopy measurements are lower in preterm infants at risk for necrotizing enterocolitis. Pediatr. Crit. Care Med. 15, 735–741 (2014).
Schat, T. E. et al. Near-infrared spectroscopy to predict the course of necrotizing enterocolitis. PLoS ONE 11, e0154710 (2016).
Le Bouhellec, J. et al. Near-infrared spectroscopy: a tool for diagnosing necrotizing enterocolitis at onset of symptoms in preterm neonates with acute gastrointestinal symptoms? Am. J. Perinatol. 38, e299–e308 (2020).
Levy, P. T. et al. Near-infrared spectroscopy for perioperative assessment and neonatal interventions. Pediatr. Res. https://doi.org/10.1038/s41390-021-01791-1 (2021).
Ostojic, D. et al. Impact of skull thickness on cerebral nirs oximetry in neonates: an in silico study. Adv. Exp. Med. Biol. 1232, 33–38 (2020).
Pichler, G., Cheung, P. Y., Tze-Fun, L., Li, E. S. & Schmolzer, G. M. Is renal tissue oxygen desaturation during severe hypoxia underestimated? An observational study in term newborn piglets. Nephrology 20, 107–109 (2015).
Ortmann, L. A. et al. Use of near-infrared spectroscopy for estimation of renal oxygenation in children with heart disease. Pediatr. Cardiol. 32, 748–753 (2011).
Dabal, R. J. et al. Inferior vena cava oxygen saturation monitoring after the norwood procedure. Ann. Thorac. Surg. 95, 2114–2120 (2013); discussion 2120–2111.
Bailey, S. M., Hendricks-Munoz, K. D. & Mally, P. Cerebral, renal, and splanchnic tissue oxygen saturation values in healthy term newborns. Am. J. Perinatol. 31, 339–344 (2014).
Bernal, N. P., Hoffman, G. M., Ghanayem, N. S. & Arca, M. J. Cerebral and somatic near-infrared spectroscopy in normal newborns. J. Pediatr. Surg. 45, 1306–1310 (2010).
Montaldo, P., De Leonibus, C., Giordano, L., De Vivo, M. & Giliberti, P. Cerebral, renal and mesenteric regional oxygen saturation of term infants during transition. J. Pediatr. Surg. 50, 1273–1277 (2015).
McNeill, S., Gatenby, J. C., McElroy, S. & Engelhardt, B. Normal cerebral, renal and abdominal regional oxygen saturations using near-infrared spectroscopy in preterm infants. J. Perinatol. 31, 51–57 (2011).
Harer, M. W. & Chock, V. Y. Renal tissue oxygenation monitoring-an opportunity to improve kidney outcomes in the vulnerable neonatal population. Front. Pediatr. 8, 241 (2020).
Terstappen, F. et al. Elevated renal tissue oxygenation in premature fetal growth restricted neonates: an observational study. PLoS ONE 13, e0204268 (2018).
Montaldo, P. et al. Impact of intrauterine growth restriction on cerebral and renal oxygenation and perfusion during the first 3 days after birth. Sci. Rep. 12, 5067 (2022).
Kleiser, S. et al. Comparison of tissue oximeters on a liquid phantom with adjustable optical properties: an extension. Biomed. Opt. Express 9, 86–101 (2018).
Ruf, B. et al. Intraoperative renal near-infrared spectroscopy indicates developing acute kidney injury in infants undergoing cardiac surgery with cardiopulmonary bypass: a case-control study. Crit. Care 19, 27 (2015).
Gist, K. M., Kwiatkowski, D. M. & Cooper, D. S. Acute kidney injury in congenital heart disease. Curr. Opin. Cardiol. 33, 101–107 (2018).
Bhalala, U. S. et al. Change in regional (somatic) near-infrared spectroscopy is not a useful indicator of clinically detectable low cardiac output in children after surgery for congenital heart defects. Pediatr. Crit. Care Med. 13, 529–534 (2012).
Jung Kim, H. et al. Acute kidney injury and renal regional oxygen saturation during aortic arch reconstruction in infants. J. Cardiothorac. Vasc. Anesth. 27, 1153–1157 (2013).
Petrova, A., Bhatt, M. & Mehta, R. Regional tissue oxygenation in preterm born infants in association with echocardiographically significant patent ductus arteriosus. J. Perinatol. 31, 460–464 (2011).
Chock, V. Y., Ramamoorthy, C. & Van Meurs, K. P. Cerebral autoregulation in neonates with a hemodynamically significant patent ductus arteriosus. J. Pediatr. 160, 936–942 (2012).
van der Laan, M. E. et al. A hemodynamically significant patent ductus arteriosus does not affect cerebral or renal tissue oxygenation in preterm infants. Neonatology 110, 141–147 (2016).
Bhatt, S. et al. Delaying cord clamping until ventilation onset improves cardiovascular function at birth in preterm lambs. J. Physiol. 591, 2113–2126 (2013).
Navikiene, J., Liubsys, A., Virsilas, E., Zvirblis, T. & Jankauskiene, A. Impact of medical treatment of hemodynamically significant patent ductus arteriosus on cerebral and renal tissue oxygenation measured by near-infrared spectroscopy in very low-birth-weight infants. Medicine 58, 475 (2022).
Guzoglu, N. et al. Renal and mesenteric tissue oxygenation in preterm infants treated with oral ibuprofen. J. Matern. Fetal Neonatal Med. 27, 197–203 (2014).
van der Laan, M. E. et al. The association between multisite near-infrared spectroscopy and routine hemodynamic measurements in relation to short-term outcome in preterms with clinical sepsis. Neonatology 108, 297–304 (2015).
van der Laan, M. E. et al. Multisite tissue oxygenation monitoring indicates organ-specific flow distribution and oxygen delivery related to low cardiac output in preterm infants with clinical sepsis. Pediatr. Crit. Care Med. 17, 764–771 (2016).
Petrova, A. & Mehta, R. Near-infrared spectroscopy in the detection of regional tissue oxygenation during hypoxic events in preterm infants undergoing critical care. Pediatr. Crit. Care Med. 7, 449–454 (2006).
Petrova, A. & Mehta, R. Regional tissue oxygenation in association with duration of hypoxaemia and haemodynamic variability in preterm neonates. Arch. Dis. Child. Fetal Neonatal Ed. 95, F213–F219 (2010).
Hoffman, S. B., Magder, L. S. & Viscardi, R. M. Renal versus cerebral saturation trajectories: the perinatal transition in preterm neonates. Pediatr. Res. 92, 1437–1442 (2022).
Bonsante, F. et al. Low renal oxygen saturation at near-infrared spectroscopy on the first day of life is associated with developing acute kidney injury in very preterm infants. Neonatology 115, 198–204 (2019).
Marin, T. & Williams, B. L. Renal oxygenation measured by near-infrared spectroscopy in neonates. Adv. Neonatal Care 21, 256–266 (2021).
Jani, P., Balegarvirupakshappa, K., Moore, J. E., Badawi, N. & Tracy, M. Regional oxygenation and perfusion monitoring to optimize neonatal packed red blood cell transfusion practices: a systematic review. Transfus. Med. Rev. 36, 27–47 (2022).
Seidel, D. et al. Changes in regional tissue oxygenation saturation and desaturations after red blood cell transfusion in preterm infants. J. Perinatol. 33, 282–287 (2013).
Aktas, S. et al. Effects of blood transfusion on regional tissue oxygenation in preterm newborns are dependent on the degree of anaemia. J. Paediatr. Child Health 55, 1209–1213 (2019).
Chock, V. Y., Frymoyer, A., Yeh, C. G. & Van Meurs, K. P. Renal saturation and acute kidney injury in neonates with hypoxic ischemic encephalopathy undergoing therapeutic hypothermia. J. Pediatr. 200, 232.e1–239.e1 (2018).
Solevag, A. L., Schmolzer, G. M., Nakstad, B., Saugstad, O. D. & Cheung, P. Y. Association between brain and kidney near-infrared spectroscopy and early postresuscitation mortality in asphyxiated newborn piglets. Neonatology 112, 80–86 (2017).
Westgarth-Taylor, C., de Lijster, L., van Bogerijen, G., Millar, A. J. & Karpelowsky, J. A prospective assessment of renal oxygenation in children undergoing laparoscopy using near-infrared spectroscopy. Surg. Endosc. 27, 3696–3704 (2013).
Holler, N. et al. Peripheral muscle near-infrared spectroscopy in neonates: ready for clinical use? A systematic qualitative review of the literature. Neonatology 108, 233–245 (2015).
Pichler, G. et al. Combination of different noninvasive measuring techniques: a new approach to increase accuracy of peripheral near infrared spectroscopy. J. Biomed. Opt. 14, 014014 (2009).
Pichler, G. et al. ‘Multi-associations’: predisposed to misinterpretation of peripheral tissue oxygenation and circulation in neonates. Physiol. Meas. 32, 1025–1034 (2011).
Hassan, I. A., Wickramasinghe, Y. A. & Spencer, S. A. Effect of limb cooling on peripheral and global oxygen consumption in neonates. Arch. Dis. Child. Fetal Neonatal Ed. 88, F139–F142 (2003).
Hassan, I. A., Wickramasinghe, Y. A. & Spencer, S. A. Effect of a change in global metabolic rate on peripheral oxygen consumption in neonates. Arch. Dis. Child. Fetal Neonatal Ed. 88, F143–F146 (2003).
Urlesberger, B. et al. Regional oxygen saturation of the brain and peripheral tissue during birth transition of term infants. J. Pediatr. 157, 740–744 (2010).
Pichler, G. et al. Forearm and calf tissue oxygenation in term neonates measured with near-infrared spectroscopy. J. Physiol. Sci. 57, 317–319 (2007).
Mileder, L. P. et al. Influence of ductus arteriosus on peripheral muscle oxygenation and perfusion in neonates. Physiol. Meas. 39, 015003 (2017).
Bay-Hansen, R., Elfving, B. & Greisen, G. Use of near infrared spectroscopy for estimation of peripheral venous saturation in newborns: comparison with co-oximetry of central venous blood. Biol. Neonate 82, 1–8 (2002).
Pichler, G. et al. Recommendations to increase the validity and comparability of peripheral measurements by near infrared spectroscopy in neonates. ‘Round table’, Section of Haematology, Oxygen Transport and Microcirculation, 48th Annual Meeting of ESPR, Prague 2007. Neonatology 94, 320–322 (2008).
Wardle, S. P., Yoxall, C. W. & Weindling, A. M. Peripheral oxygenation in hypotensive preterm babies. Pediatr. Res. 45, 343–349 (1999).
Kissack, C. M. & Weindling, A. M. Peripheral blood flow and oxygen extraction in the sick, newborn very low birth weight infant shortly after birth. Pediatr. Res. 65, 462–467 (2009).
Victor, S., Marson, A. G., Appleton, R. E., Beirne, M. & Weindling, A. M. Relationship between blood pressure, cerebral electrical activity, cerebral fractional oxygen extraction, and peripheral blood flow in very low birth weight newborn infants. Pediatr. Res. 59, 314–319 (2006).
Pichler, G. et al. C reactive protein: impact on peripheral tissue oxygenation and perfusion in neonates. Arch. Dis. Child., Fetal Neonatal Ed. 97, F444–F448 (2012).
Tax, N. et al. The influence of perinatal asphyxia on peripheral oxygenation and perfusion in neonates. Early Hum. Dev. 89, 483–486 (2013).
Milan, A. et al. Near-infrared spectroscopy measure of limb peripheral perfusion in neonatal arterial thromboembolic disease. Minerva Pediatr. 64, 633–639 (2012).
Baenziger, O., Keel, M., Bucher, H. U. & Wolf, M. Oxygen extraction index measured by near infrared spectroscopy—a parameter for monitoring tissue oxygenation? Adv. Exp. Med. Biol. 645, 161–166 (2009).
Zaramella, P. et al. Early versus late cord clamping: effects on peripheral blood flow and cardiac function in term infants. Early Hum. Dev. 84, 195–200 (2008).
Redlin, M. et al. Minimizing intraoperative hemodilution by use of a very low priming volume cardiopulmonary bypass in neonates with transposition of the great arteries. J. Thorac. Cardiovasc. Surg. 142, 875–881 (2011).
Redlin, M. et al. How near-infrared spectroscopy differentiates between lower body ischemia due to arterial occlusion versus venous outflow obstruction. Ann. Thorac. Surg. 91, 1274–1276 (2011).
Pellicer, A. et al. Phase 1 study of two inodilators in neonates undergoing cardiovascular surgery. Pediatr. Res. 73, 95–103 (2013).
Wardle, S. P., Garr, R., Yoxall, C. W. & Weindling, A. M. A pilot randomised controlled trial of peripheral fractional oxygen extraction to guide blood transfusions in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 86, F22–F27 (2002).
Pichler, G. et al. Avoiding arterial hypotension in preterm neonates (AHIP)-a single center randomised controlled study investigating simultaneous near infrared spectroscopy measurements of cerebral and peripheral regional tissue oxygenation and dedicated interventions. Front. Pediatr. 6, 15 (2018).
Teller, J. et al. Can near infrared spectroscopy of the liver monitor tissue oxygenation? Eur. J. Pediatr. 159, 549 (2000).
Dani, C. et al. Measurement of lung oxygenation by near-infrared spectroscopy in preterm infants with respiratory distress syndrome: a proof-of-concept study. Pediatr. Pulmonol. 57, 2306–2312 (2022).
Schwartz, A. E. et al. Cerebral blood flow is determined by arterial pressure and not cardiopulmonary bypass flow rate. Ann. Thorac. Surg. 60, 165–169 (1995); d iscussion 169–170.
Michler, R. E., Sandhu, A. A., Young, W. L. & Schwartz, A. E. Low-flow cardiopulmonary bypass: importance of blood pressure in maintaining cerebral blood flow. Ann. Thorac. Surg. 60, S525–S528 (1995).
Bouma, G. J. & Muizelaar, J. P. Relationship between cardiac output and cerebral blood flow in patients with intact and with impaired autoregulation. J. Neurosurg. 73, 368–374 (1990).
Eicke, B. M. et al. Lack of association between carotid artery volume blood flow and cardiac output. J. Ultrasound Med. 20, 1293–1298 (2001); quiz 1300.
Crean, P. A. et al. The fractional distribution of the cardiac output in man using microspheres labelled with technetium 99m. Br. J. Radiol. 59, 209–215 (1986).
Rhee, C. J. et al. Renovascular reactivity measured by near-infrared spectroscopy. J. Appl. Physiol. 113, 307–314 (2012).
Kluckow, M. & Evans, N. Relationship between blood pressure and cardiac output in preterm infants requiring mechanical ventilation. J. Pediatr. 129, 506–512 (1996).
Morales, D. L. et al. Restoration of renal function in shock by perfusion of the renal artery with venous blood: a counterintuitive approach. Crit. Care Med. 30, 1297–1300 (2002).
Hoeller, N. et al. Cerebral and peripheral muscle oxygenation and perfusion: course in moderate and late preterm neonates during the first day after birth. Physiol. Int. 107, 267–279 (2020).
Hanson, S. J., Berens, R. J., Havens, P. L., Kim, M. K. & Hoffman, G. M. Effect of volume resuscitation on regional perfusion in dehydrated pediatric patients as measured by two-site near-infrared spectroscopy. Pediatr. Emerg. Care 25, 150–153 (2009).
Chakravarti, S. B. et al. Multisite near-infrared spectroscopy predicts elevated blood lactate level in children after cardiac surgery. J. Cardiothorac. Vasc. Anesth. 23, 663–667 (2009).
Fortune, P. M., Wagstaff, M. & Petros, A. J. Cerebro-splanchnic oxygenation ratio (CSOR) using near infrared spectroscopy may be able to predict splanchnic ischaemia in neonates. Intensive Care Med. 27, 1401–1407 (2001).
Dave, V. et al. Splanchnic tissue oxygenation, but not brain tissue oxygenation, increases after feeds in stable preterm neonates tolerating full bolus orogastric feeding. J. Perinatol. 29, 213–218 (2009).
Braski, K. et al. Splanchnic-cerebral oxygenation ratio decreases during enteral feedings in anemic preterm infants: observations under near-infrared spectroscopy. Neonatology 113, 75–80 (2018).
Bozzetti, V. et al. Cerebral and somatic NIRS-determined oxygenation in iugr preterm infants during transition. J. Matern. Fetal Neonatal Med. 29, 443–446 (2016).
Mebius, M. J. et al. Near-infrared spectroscopy as a predictor of clinical deterioration: a case report of two infants with duct-dependent congenital heart disease. BMC Pediatr. 17, 79 (2017).
van der Laan, M. E. et al. Cerebral and renal oxygen saturation are not compromised in the presence of retrograde blood flow in either the ascending or descending aorta in term or near-term infants with left-sided obstructive lesions. Neonatology 112, 217–224 (2017).
Terstappen, F. et al. Prenatal use of sildenafil in fetal growth restriction and its effect on neonatal tissue oxygenation-a retrospective analysis of hemodynamic data from participants of the Dutch Strider Trial. Front. Pediatr. 8, 595693 (2020).
Grossauer, K. et al. Comparison of peripheral and cerebral tissue oxygenation index in neonates. Arch. Dis. Child. Fetal Neonatal Ed. 94, F156 (2009).
Pellicer, A. et al. The SafeBoosC phase II randomised clinical trial: a treatment guideline for targeted near-infrared-derived cerebral tissue oxygenation versus standard treatment in extremely preterm infants. Neonatology 104, 171–178 (2013).
Hyttel-Sorensen, S. et al. A phase II randomized clinical trial on cerebral near-infrared spectroscopy plus a treatment guideline versus treatment as usual for extremely preterm infants during the first three days of life (SafeBoosC): study protocol for a randomized controlled trial. Trials 14, 120 (2013).
Hansen, M. L. et al. Cerebral near-infrared spectroscopy monitoring versus treatment as usual for extremely preterm infants: a protocol for the SafeBoosC randomised clinical phase III trial. Trials 20, 811 (2019).
Funding
Financial support of publication costs by the European Society for Pediatric Research (ESPR) is gratefully acknowledged.
Author information
Authors and Affiliations
Consortia
Contributions
Each author has contributed to this work. E.M.W.K., J.P.M., C.J.R., E.E., C.E.S., and G.P. each drafted parts of the article and revised the manuscript critically for important intellectual content. All authors revised the manuscript critically for important intellectual content and gave final approval of the version to be published. All members of the European Special Interest Group “Near-InfraRed Spectroscopy” who actively participated in the creation of this manuscript are listed in the appendix. All these members have substantially contributed to the conception and revision of the manuscript and approved the final version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kooi, E.M.W., Mintzer, J.P., Rhee, C.J. et al. Neonatal somatic oxygenation and perfusion assessment using near-infrared spectroscopy. Pediatr Res (2024). https://doi.org/10.1038/s41390-024-03226-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-024-03226-z