Abstract
Background
Neonatal encephalopathy (NE) remains a common cause of infant morbidity and mortality. Neuropathological corollaries of NE associated with acute hypoxia-ischemia include a central injury pattern involving the basal ganglia and thalamus, which may interfere with thermoregulatory circuits. Spontaneous hypothermia (SH) occurs in both preclinical models and clinical hypoxic-ischemic NE and may provide an early biomarker of injury severity. To determine whether SH predicts the degree of injury in a ferret model of hypoxic-ischemic NE, we investigated whether rectal temperature (RT) 1 h after insult correlated with long-term outcomes.
Methods
Postnatal day (P)17 ferrets were presensitized with Escherichia coli lipopolysaccharide before undergoing hypoxia-ischemia/hyperoxia (HIH): bilateral carotid artery ligation, hypoxia-hyperoxia-hypoxia, and right ligation reversal. One hour later, nesting RTs were measured.
Results
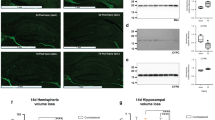
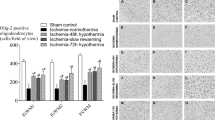
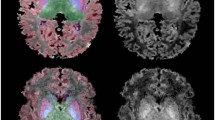
Animals exposed to HIH were separated into normothermic (NT; ≥34.4 °C) or spontaneously hypothermic (SH; <34.4 °C) groups. At P42, cortical development, ex vivo MRI, and neuropathology were quantitated. Whole-brain volume and fractional anisotropy in SH brains were significantly decreased compared to control and NT animals. SH brains also had significantly altered gyrification, greater cortical pathology, and increased corpus callosum GFAP staining relative to NT and control brains.
Conclusion
In near-term-equivalent ferrets, nesting RT 1 h after HIH may predict long-term neuropathological outcomes.
Impact
-
High-throughput methods to determine injury severity prior to treatment in animal studies of neonatal brain injury are lacking.
-
In a gyrified animal model of neonatal inflammation-sensitized hypoxic-ischemic brain injury in the ferret, rectal temperature 1 h after hypoxia predicts animals who will have increased cortical pathology and white matter changes on MRI.
-
These changes parallel similar responses in rodents and humans but have not previously been correlated with long-term neuropathological outcomes in gyrified animal models.
-
Endogenous thermoregulatory responses to injury may provide a translational marker of injury severity to help stratify animals to treatment groups or predict outcome in preclinical studies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Organization WH. Newborn Mortality. https://www.who.int/news-room/fact-sheets/detail/levels-and-trends-in-child-mortality-report-2021 (2022).
Patel, S. D. et al. Therapeutic hypothermia and hypoxia-ischemia in the term-equivalent neonatal rat: characterization of a translational preclinical model. Pediatr. Res. 78, 264–271, https://doi.org/10.1038/pr.2015.100 (2015).
Thayyil, S. et al. Hypothermia for moderate or severe neonatal encephalopathy in low-income and middle-income countries (HELIX): a randomised controlled trial in India, Sri Lanka, and Bangladesh. Lancet Glob. Health 9, e1273–e1285, https://doi.org/10.1016/S2214-109X(21)00264-3 (2021).
Jacobs, S. E. et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst. Rev. 1, Cd003311, https://doi.org/10.1002/14651858.CD003311.pub3 (2013).
Zhou, K. Q., Davidson, J. O., Bennet, L. & Gunn, A. J. Combination treatments with therapeutic hypothermia for hypoxic-ischemic neuroprotection. Dev. Med. Child Neurol. 62, 1131–1137, https://doi.org/10.1111/dmcn.14610 (2020).
Victor, S. et al. New possibilities for neuroprotection in neonatal hypoxic-ischemic encephalopathy. Eur. J. Pediatr. 181, 875–887, https://doi.org/10.1007/s00431-021-04320-8 (2022).
O'Mara, K. & McPherson, C. Neuroprotective agents for neonates with hypoxic-ischemic encephalopathy. Neonatal. Netw. 40, 406–413, https://doi.org/10.1891/11-T-755 (2021).
Wu, Y. W. et al. Trial of erythropoietin for hypoxic-ischemic encephalopathy in newborns. N. Engl. J. Med. 387, 148–159, https://doi.org/10.1056/NEJMoa2119660 (2022).
Kao, Y. J. et al. Early neuroimaging and ultrastructural correlates of injury outcome after neonatal hypoxic-ischaemia. Brain Commun. 3, fcab048, https://doi.org/10.1093/braincomms/fcab048 (2021).
Wood, T. et al. Rectal temperature in the first five hours after hypoxia-ischemia critically affects neuropathological outcomes in neonatal rats. Pediatr. Res. 83, 536–544, https://doi.org/10.1038/pr.2017.51 (2018).
Busto, R. et al. Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J. Cereb Blood Flow Metab. 7, 729–738, https://doi.org/10.1038/jcbfm.1987.127 (1987).
Enweronu-Laryea, C. et al. Core temperature after birth in babies with neonatal encephalopathy in a sub-Saharan African hospital setting. J. Physiol. 597, 4013–4024, https://doi.org/10.1113/JP277820 (2019).
Flibotte, J. et al. Blanket temperature during therapeutic hypothermia and outcomes in hypoxic ischemic encephalopathy. J. Perinatol. 42, 348–353, https://doi.org/10.1038/s41372-021-01302-4 (2022).
Mietzsch, U. et al. Active cooling temperature required to achieve therapeutic hypothermia correlates with short-term outcome in neonatal hypoxic-ischaemic encephalopathy. J. Physiol. 598, 415–424, https://doi.org/10.1113/JP278790 (2020).
Mietzsch U, et al. Temperature dysregulation during therapeutic hypothermia predicts long-term outcome in neonates with HIE. J. Cereb. Blood Flow Metab. 2023. In Press.
Wu, T. W. et al. Cerebral lactate concentration in neonatal hypoxic-ischemic encephalopathy: in relation to time, characteristic of injury, and serum lactate concentration. Front. Neurol. 9, 293, https://doi.org/10.3389/fneur.2018.00293 (2018).
Morrison, S. F., Nakamura, K. & Madden, C. J. Central control of thermogenesis in mammals. Exp. Physiol. 93, 773–797, https://doi.org/10.1113/expphysiol.2007.041848. (2008).
Utter, A. A. & Basso, M. A. The basal ganglia: an overview of circuits and function. Neurosci. Biobehav. Rev. 32, 333–342, https://doi.org/10.1016/j.neubiorev.2006.11.003 (2008).
Burnard, E. D. & Cross, K. W. Rectal temperature in the newborn after birth asphyxia. Br. Med. J. 2, 1197–1199, https://doi.org/10.1136/bmj.2.5106.1197 (1958).
Jayasinghe, D. Innate hypothermia after hypoxic ischaemic delivery. Neonatology 107, 220–223, https://doi.org/10.1159/000369119 (2015).
Corry KA, et al. Evaluating neuroprotective effects of uridine, erythropoietin, and therapeutic hypothermia in a ferret model of inflammation-sensitized hypoxic-ischemic encephalopathy. Int. J. Mol. Sci. 22 (2021). https://doi.org/10.3390/ijms22189841
Wood T, et al. A ferret model of inflammation-sensitized late preterm hypoxic-ischemic brain injury. J. Vis. Exp. (153) (2019). https://doi.org/10.3791/60131
R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical computing. (2019).
Wieshmann, U. C. et al. Reduced anisotropy of water diffusion in structural cerebral abnormalities demonstrated with diffusion tensor imaging. Magn. Reson. Imaging. 17, 1269–1274, https://doi.org/10.1016/s0730-725x(99)00082-x (1999).
Vijayan, V. K., Lee, Y. L. & Eng, L. F. Increase in glial fibrillary acidic protein following neural trauma. Mol. Chem. Neuropathol. 13, 107–118, https://doi.org/10.1007/BF03159912 (1990).
Liu, Y., Silverstein, F. S., Skoff, R. & Barks, J. D. Hypoxic-ischemic oligodendroglial injury in neonatal rat brain. Pediatr. Res. 51, 25–33, https://doi.org/10.1203/00006450-200201000-00007 (2002).
Ginsberg, M. D. et al. Therapeutic modulation of brain temperature: relevance to ischemic brain injury. Cerebrovasc Brain Metab. Rev. 4, 189–225 (1992).
Jang, S. H. & Kwon, H. G. Injury of the hypothalamus in patients with hypoxic-ischemic brain injury: a diffusion tensor imaging study. Am. J. Phys. Med. Rehabil. 97, 160–163, https://doi.org/10.1097/PHM.0000000000000813 (2018).
Hutchinson, E. B. et al. Population based MRI and DTI templates of the adult ferret brain and tools for voxelwise analysis. Neuroimage 152, 575–589, https://doi.org/10.1016/j.neuroimage.2017.03.009 (2017).
Gluckman, P. D. et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 365, 663–670, https://doi.org/10.1016/S0140-6736(05)17946-X (2005).
Tsuda, K. et al. Body temperature, heart rate, and short-term outcome of cooled infants. Ther. Hypothermia Temp. Manag. 9, 76–85, https://doi.org/10.1089/ther.2018.0019 (2019).
Falck, M. et al. Hypothermic neuronal rescue from infection-sensitised hypoxic-ischaemic brain injury is pathogen dependent. Dev. Neurosci. 39, 238–247, https://doi.org/10.1159/000455838 (2017).
Falck, M. et al. Neonatal systemic inflammation induces inflammatory reactions and brain apoptosis in a pathogen-specific manner. Neonatology 113, 212–220, https://doi.org/10.1159/000481980 (2018).
Osredkar, D. et al. Hypothermia does not reverse cellular responses caused by lipopolysaccharide in neonatal hypoxic-ischaemic brain injury. Dev. Neurosci. 37, 390–397, https://doi.org/10.1159/000430860 (2015).
Acknowledgements
The authors would like to thank Simar Virk and Annamarie Shearlock for assisting in brain measurements and preparing brains for MRI.
Funding
This work was funded by the Bill and Melinda Gates Foundation.
Author information
Authors and Affiliations
Contributions
Data acquisition: O.R.W., K.A.C., D.H.M., J.B.L., J.M.S., T.R.W. Data analysis: O.R.W., T.R.W. Interpretation: O.R.W., U.M., S.E.J., T.R.W. Manuscript drafting: O.R.W., T.R.W. Manuscript editing and revision: O.R.W., K.A.C., D.H.M., J.B.L., J.M.S., U.M., S.E.J.,T.R.W. Approval of final manuscript: O.R.W., K.A.C., D.H.M., J.B.L., J.M.S., U.M., S.E.J., T.R.W.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
White, O.R., Corry, K.A., Moralejo, D.H. et al. Rectal temperature after hypoxia-ischemia predicts white matter and cortical pathology in the near-term ferret. Pediatr Res 95, 84–92 (2024). https://doi.org/10.1038/s41390-023-02793-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02793-x