Abstract
Background
Extremely low gestational age neonates (ELGANs) are at risk for chronic kidney disease. The long-term kidney effects of neonatal caffeine are unknown. We hypothesize that prolonged caffeine exposure will improve kidney function at 22–26 months.
Methods
Secondary analysis of the Preterm Erythropoietin Neuroprotection Trial of neonates <28 weeks’ gestation. Participants included if any kidney outcomes were collected at 22–26 months corrected age. Exposure was post-menstrual age of caffeine discontinuation. Primary outcomes: ‘reduced eGFR’ <90 ml/min/1.73 m2, ‘albuminuria’ (>30 mg albumin/g creatinine), or ‘elevated blood pressure’ (BP) >95th %tile. A general estimating equation logistic regression model stratified by bronchopulmonary dysplasia (BPD) status was used.
Results
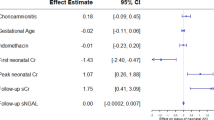
598 participants had at least one kidney metric at follow up. Within the whole cohort, postmenstrual age of caffeine discontinuation was not associated with any abnormal measures of kidney function at 2 years. In the stratified analysis, for each additional week of caffeine, the no BPD group had a 21% decreased adjusted odds of eGFR <90 ml/min/1.73m2 (aOR 0.78; CI 0.62–0.99) and the BPD group had a 15% increased adjusted odds of elevated BP (aOR 1.15; CI: 1.05–1.25).
Conclusions
Longer caffeine exposure during the neonatal period is associated with differential kidney outcomes at 22–26 months dependent on BPD status.
Impact
-
In participants born <28 weeks’ gestation, discontinuation of caffeine at a later post menstrual age was not associated with abnormal kidney outcomes at 22–26 months corrected age.
-
When assessed at 2 years of age, later discontinuation of caffeine in children born <28 weeks’ gestation was associated with a greater risk of reduced eGFR in those without a history of BPD and an increased odds of hypertension in those with a history of BPD.
-
More work is necessary to understand the long-term impact of caffeine on the developing kidney.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets analyzed during the current study can be requested from the NINDS at the following website: https://www.ninds.nih.gov/current-research/research-funded-ninds/clinical-research/archived-clinical-research-datasets.
References
Charlton, J. R., Baldelomar, E. J., Hyatt, D. M. & Bennett, K. M. Nephron number and its determinants: a 2020 update. Pediatr. Nephrol. 36, 797–807 (2021).
Jetton, J. G. et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc. Health 1, 184–194 (2017).
Askenazi, D. J. et al. Prevalence of acute kidney injury (AKI) in extremely low gestational age neonates (ELGAN). Pediatr. Nephrol. 35, 1737–1748 (2020).
Coca, S. G., Singanamala, S. & Parikh, C. R. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int 81, 442–448 (2012).
Mammen, C. et al. Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am. J. Kidney Dis. 59, 523–530 (2012).
Crump, C., Sundquist, J., Winkleby, M. A. & Sundquist, K. Preterm birth and risk of chronic kidney disease from childhood into mid-adulthood: national cohort study. BMJ 365, l1346 (2019).
Harer, M. W., Pope, C. F., Conaway, M. R. & Charlton, J. R. Follow-up of acute kidney injury in neonates during childhood years (FANCY): a prospective cohort study. Pediatr. Nephrol. 32, 1067–1076 (2017).
Bruel, A. et al. Renal outcome in children born preterm with neonatal acute renal failure: ireneo—a prospective controlled study. Pediatr. Nephrol. 31, 2365–2373 (2016).
Hingorani, S. et al. Prevalence and risk factors for kidney disease and elevated BP in 2-year-old children born extremely premature. Clin. J. Am. Soc. Nephrol. 17, 1129–1138 (2022).
Adebayo, O. C. et al. Glomerular hyperfiltration: part 2—clinical significance in children. Pediatr. Nephrol. 38, 2529–2547 (2023).
Ji, D. et al. Wide variation in caffeine discontinuation timing in premature infants. J. Perinatol. 40, 288–293 (2020).
Harer, M. W. et al. Association between early caffeine citrate administration and risk of acute kidney injury in preterm neonates: results from the AWAKEN study. JAMA Pediatr. 172, e180322 (2018).
Al-Wassia, H., Alshaikh, B. & Sauve, R. Prophylactic theophylline for the prevention of severe renal dysfunction in term and post-term neonates with perinatal asphyxia: a systematic review and meta-analysis of randomized controlled trials. J. Perinatol. 33, 271–277 (2013).
Bhatt, G. C., Gogia, P., Bitzan, M. & Das, R. R. Theophylline and aminophylline for prevention of acute kidney injury in neonates and children: a systematic review. Arch. Dis. Child 104, 670–679 (2019).
Schmidt, B. et al. Survival without disability to age 5 years after neonatal caffeine therapy for apnea of prematurity. JAMA 307, 275–282 (2012).
Schmidt, B. et al. Academic performance, motor function, and behavior 11 years after neonatal caffeine citrate therapy for apnea of prematurity: an 11-year follow-up of the CAP randomized clinical trial. JAMA Pediatr. 171, 564–572 (2017).
Juul, S. E. et al. A randomized trial of erythropoietin for neuroprotection in preterm infants. N. Engl. J. Med. 382, 233–243 (2020).
Juul, S. E., Mayock, D. E., Comstock, B. A. & Heagerty, P. J. Neuroprotective potential of erythropoietin in neonates; design of a randomized trial. Matern Health Neonatol. Perinatol. 1, 27 (2015).
Askenazi, D. J. et al. The impact of erythropoietin on short- and long-term kidney-related outcomes in neonates of extremely low gestational age. results of a multicenter, double-blind, placebo-controlled randomized clinical trial. J. Pediatr. 232, 65–72.e67 (2021).
Flynn, J. T. et al. Subcommittee on Screening and Management of High Blood Pressure in Children. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics 140, e20171904 (2017).
Pierce, C. B. et al. Age- and sex-dependent clinical equations to estimate glomerular filtration rates in children and young adults with chronic kidney disease. Kidney Int. 99, 948–956 (2021).
Fuhrman, D. Y. et al. Albuminuria, proteinuria, and renal disease progression in children with CKD. Clin. J. Am. Soc. Nephrol. 12, 912–920 (2017).
Schmidt, B. et al. Long-term effects of caffeine therapy for apnea of prematurity. N. Engl. J. Med. 357, 1893–1902 (2007).
Davis, P. G. et al. Caffeine for apnea of prematurity trial: benefits may vary in subgroups. J. Pediatr. 156, 382–387 (2010).
Dobson, N. R. et al. Trends in caffeine use and association between clinical outcomes and timing of therapy in very low birth weight infants. J. Pediatr. 164, 992–998.e993 (2014).
Lodha, A. et al. Association of early caffeine administration and neonatal outcomes in very preterm neonates. JAMA Pediatr. 169, 33–38 (2015).
Lodha, A. et al. Does duration of caffeine therapy in preterm infants born ≤1250 G at birth influence neurodevelopmental (ND) outcomes at 3 years of age? J. Perinatol. 38, 889–899 (2018).
Starr, M. C. & Wilson, A. C. Systemic hypertension in infants with bronchopulmonary dysplasia. Curr. Hypertens. Rep. 24, 193–203 (2022).
De Giuseppe, R., Di Napoli, I., Granata, F., Mottolese, A. & Cena, H. Caffeine and blood pressure: a critical review perspective. Nutr. Res Rev. 32, 169–175 (2019).
Srithongkul, T. & Ungprasert, P. Coffee consumption is associated with a decreased risk of incident chronic kidney disease: a systematic review and meta-analysis of cohort studies. Eur. J. Intern Med. 77, 111–116 (2020).
Tommerdahl, K. L. et al. Coffee consumption may mitigate the risk for acute kidney injury: results from the atherosclerosis risk in communities study. Kidney Int. Rep. 7, 1665–1672 (2022).
Di Fiore, J. M., MacFarlane, P. M. & Martin, R. J. Intermittent hypoxemia in preterm infants. Clin. Perinatol. 46, 553–565 (2019).
Raffay, T. M. et al. Neonatal intermittent hypoxemia events are associated with diagnosis of bronchopulmonary dysplasia at 36 weeks postmenstrual age. Pediatr. Res. 85, 318–323 (2019).
Zangaladze, A., Cai, C. L., Marcelino, M., Aranda, J. V. & Beharry, K. D. Renal biomarkers of acute kidney injury in response to increasing intermittent hypoxia episodes in the neonatal rat. BMC Nephrol. 22, 299 (2021).
Beaudin, A. E. et al. Risk of chronic kidney disease in patients with obstructive sleep apnea. Sleep 45, zsab267 (2022).
Rhein, L. M. et al. Effects of caffeine on intermittent hypoxia in infants born prematurely: a randomized clinical trial. JAMA Pediatr. 168, 250–257 (2014).
Heilbron, D. C., Holliday, M. A., al-Dahwi, A. & Kogan, B. A. Expressing glomerular filtration rate in children. Pediatr. Nephrol. 5, 5–11 (1991).
Funding
The study is coordinated by the Neonatal Kidney Collaborative Research Committee. Statistical support for this study is provided by Nuwellis. The original study, the Preterm Erythropoietin Neuroprotection Trial (PENUT trial) was funded by the NIH-NINDS (U01 NS077953, U01 NS077955). Kidney-specific data collection was funded by the NIH-NIDDK as part of the Recombinant Erythropoietin for Protection of Infant Renal Disease (REPaIReD) Study (NIH NIDDK (R01 DK103608).
Author information
Authors and Affiliations
Consortia
Contributions
M.W.H. made significant contributions to the design and conception, data analysis and interpretation, drafting the manuscript, critical revision of the manuscript and final approval. R.Griffin made significant contributions to the statistical analysis and interpretation, drafting the manuscript, and final approval. D.A. made significant contributions to the design, data interpretation, critical revision of the manuscript and final approval. M.F. made contributions to the design of the study, revision of the manuscript and final approval. R.Guillet made significant contributions to the design and conception, data interpretation, critical revision of the manuscript and final approval. M.H. made contributions to the design of the study, revision of the manuscript and final approval. M.S. made contributions to the design of the study, revision of the manuscript and final approval. C.S. made significant contributions to the design of the study, revision of the manuscript and final approval. R.W. made significant contributions to the design, data interpretation, critical revision of the manuscript and final approval. J.R.C. made significant contributions to the design and conception, data analysis and interpretation, critical revision of the manuscript and final approval.
Corresponding author
Ethics declarations
Competing interests
All authors declare no real or perceived conflicts of interest that could affect the study design, collection, analysis, and interpretation of data, writing of the report, or the decision to submit for publication. For full disclosure, we provide here an additional list of other author’s commitments and funding sources that are not directly related to this study: M.W.H. receives research funding unrelated to this project from the NIH, Wisconsin Partnership Program, and Meriter Foundation. D.J.A. is a consultant for Baxter, Nuwellis, Medtronic Bioporto, and Seastar. His institution receives grant funding for education and research that is not related to this project from NIH, Baxter, Nuwellis, Medtronic, Bioporto, and Seastar. He has patents pending on inventions to improve the kidney care of neonates. He is the Founder and Chief Scientific Officer for Zorro-Flow. J.R.C. is a consultant for Medtronics and investor in Zorro-Flow. She receives funding for research not related to this project from the NIH. She is Vice-President of the Neonatal Kidney Collaborative. R.Guillet is a consultant for NEMA Research. She receives funding for research not related to this project from NIH. C.S. is a consultant for AM Pharma which is unrelated to the content in this manuscript. Meredith Schuh receives research funding unrelated to this project from NIH and Otsuka
Ethics approval and consent to participate
Consent was required for parent study but not for this secondary analysis.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Harer, M.W., Griffin, R., Askenazi, D.J. et al. Caffeine and kidney function at two years in former extremely low gestational age neonates. Pediatr Res 95, 257–266 (2024). https://doi.org/10.1038/s41390-023-02792-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02792-y