Abstract
Background
Many drugs are used off-label or unlicensed in neonates. This does not mean they are used without evidence or knowledge. We aimed to apply and evaluate the Grading and Assessment of Pharmacokinetic–Pharmacodynamic Studies (GAPPS) scoring system for the level of evidence of two commonly used anti-epileptic drugs.
Methods
Midazolam and phenobarbital as anti-epileptics were evaluated with a systematic literature search on neonatal pharmacokinetic (PK) and/or pharmacodynamic [PD, (amplitude-integrated) electroencephalography effect] studies. With the GAPPS system, two evaluators graded the current level of evidence. Inter-rater agreement was assessed for dosing evidence score (DES), quality of evidence (QoE), and strength of recommendation (REC).
Results
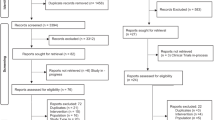
Seventy-two studies were included. DES scores 4 and 9 were most frequently used for PK, and scores 0 and 1 for PD. Inter-rater agreements on DES, QoE, and REC ranged from moderate to very good. A final REC was provided for all PK studies, but only for 25% (midazolam) and 33% (phenobarbital) of PD studies.
Conclusions
There is a reasonable level of evidence concerning midazolam and phenobarbital PK in neonates, although using a predefined target without integrated PK/PD evaluation. Further research is needed on midazolam use in term neonates with therapeutic hypothermia, and phenobarbital treatment in preterms.
Impact
-
There is a reasonable level of evidence concerning pharmacotherapy of midazolam and phenobarbital in neonates. Most evidence is however based on PK studies, using a predefined target level or concentration range without integrated, combined PK/PD evaluation.
-
Using the GAPPS system, final strength of recommendation could be provided for all PK studies, but only for 25% (midazolam) to 33% (phenobarbital) of PD studies.
-
Due to the limited PK observations of midazolam in term neonates with therapeutic hypothermia, and of phenobarbital in preterm neonates these subgroups can be identified for further research.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its Supplementary Information files. For additional information concerning these data, the corresponding author can be contacted.
References
Flint, R. B. et al. Large differences in neonatal drug use between nicus are common practice: time for consensus? Br. J. Clin. Pharmacol. 84, 1313–1323 (2018).
Slater, R., Moultrie, F., Bax, R., van den Anker, J. & Bhatt, A. Preterm health: time to bridge the evidence gap. Lancet 396, 872–873 (2020).
van den Anker, J., Reed, M. D., Allegaert, K. & Kearns, G. L. Developmental changes in pharmacokinetics and pharmacodynamics. J. Clin. Pharmacol. 58, S10–S25 (2018).
Smits, A. et al. Current knowledge, challenges and innovations in developmental pharmacology: a combined Conect4children Expert Group and European Society for Developmental, Perinatal and Paediatric Pharmacology White Paper. Br. J. Clin. Pharmacol. 88, 4965–4984 (2022).
Smits, A. et al. Prospective evaluation of a model-based dosing regimen for amikacin in preterm and term neonates in clinical practice. Antimicrob. Agents Chemother. 59, 6344–6351 (2015).
Smits, A., Kulo, A., van den Anker, J. & Allegaert, K. The amikacin research program: a stepwise approach to validate dosing regimens in neonates. Expert Opin. Drug Metab. Toxicol. 13, 157–166 (2017).
Atkins, D. et al. Grading quality of evidence and strength of recommendations. BMJ 328, 1490 (2004).
Gastine, S. et al. GAPPS (Grading and Assessment of Pharmacokinetic-Pharmacodynamic Studies) a critical appraisal system for antimicrobial PKPD studies - development and application in pediatric antibiotic studies. Expert Rev. Clin. Pharmacol. 12, 1091–1098 (2019).
Cohen, J. Weighted Kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol. Bull. 70, 213–220 (1968).
Altman, D. G. Practical Statistics for Medical Research (Chapman & Hall/CRC, 1991).
Engbers, A. G. J. et al. Enantiomer specific pharmacokinetics of ibuprofen in preterm neonates with patent ductus arteriosus. Br. J. Clin. Pharmacol. 86, 2028–2039 (2020).
Engbers, A. G. J. et al. The pharmacokinetics of caffeine in preterm newborns: no influence of doxapram but important maturation with age. Neonatology 118, 106–113 (2021).
Voller, S. et al. Recently registered midazolam doses for preterm neonates do not lead to equal exposure: a population pharmacokinetic model. J. Clin. Pharmacol. 59, 1300–1308 (2019).
Shaniv, D. et al. Neonatal drug formularies-a global scope. Children 10, 848 (2023).
Barker, C. I. S. et al. Pharmacokinetic studies in children: recommendations for practice and research. Arch. Dis. Child. 103, 695–702 (2018).
van den Broek, M. P. et al. Pharmacokinetics and clinical efficacy of phenobarbital in asphyxiated newborns treated with hypothermia: a thermopharmacological approach. Clin. Pharmacokinet. 51, 671–679 (2012).
Nijstad, A. L. et al. Clinical pharmacology of cytotoxic drugs in neonates and infants: providing evidence-based dosing guidance. Eur. J. Cancer 164, 137–154 (2022).
van der Zanden, T. M. et al. Developing a paediatric drug formulary for the Netherlands. Arch. Dis. Child. 102, 357–361 (2017).
van der Zanden, T. M. et al. Extending the Dutch Paediatric Formulary across Europe: successful development of country specific, parallel, paediatric drug formularies. Abstract ESDPPP Congres Basel, Switserland. Arch. Dis. Child. 104, e59–e60 (2019).
van der Zanden, T. M. et al. Off-label, but on-evidence? A review of the level of evidence for pediatric pharmacotherapy. Clin. Pharmacol. Ther. 112, 1243–1253 (2022).
Kanji, S. et al. Reporting guidelines for clinical pharmacokinetic studies: the ClinPK Statement. Clin. Pharmacokinet. 54, 783–795 (2015).
Schmidt, B. et al. Long-term effects of caffeine therapy for apnea of prematurity. N. Engl. J. Med. 357, 1893–1902 (2007).
Schmidt, B. et al. Survival without disability to age 5 years after neonatal caffeine therapy for apnea of prematurity. JAMA 307, 275–282 (2012).
Koch, G. et al. Caffeine citrate dosing adjustments to assure stable caffeine concentrations in preterm neonates. J. Pediatr. 191, 50.e1–56.e1 (2017).
Allegaert, K. Rational use of medicines in neonates: current observations, areas for research and perspectives. Healthcare 6, 115 (2018).
Allegaert, K., Smits, A., Simons, S. & den Anker, J. V. Perspectives in neonatal pharmacology: drug discovery, knowledge integration and structured prioritization. Curr. Pharm. Des. 24, 4839–4841 (2018).
Soul, J. S. et al. Recommendations for the design of therapeutic trials for neonatal seizures. Pediatr. Res. 85, 943–954 (2019).
Young, L., Berg, M. & Soll, R. Prophylactic barbiturate use for the prevention of morbidity and mortality following perinatal asphyxia. Cochrane Database Syst. Rev. 2016, CD001240 (2016).
Glass, H. C. et al. Safety of early discontinuation of antiseizure medication after acute symptomatic neonatal seizures. JAMA Neurol. 78, 817–825 (2021).
Kumar, J., Meena, J., Yadav, J. & Saini, L. Efficacy and safety of phenobarbitone as first-line treatment for neonatal seizure: a systematic review and meta-analysis. J. Trop. Pediatr. 67, fmab008 (2021).
FDA. U.S. Food and Drug Administration. NDA approval letter phenobarbital sodium. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/215910Orig1s000ltr.pdf (2022).
Sun Pharmaceutical Industries. Sezaby (phenobarbital sodium) for injection. https://sezaby.com/ (2023).
Sharpe, C. et al. Levetiracetam versus phenobarbital for neonatal seizures: a randomized controlled trial. Pediatrics 145, e20193182 (2020).
Cuzzolin, L. & Agostino, R. Off-label and unlicensed drug treatments in neonatal intensive care units: an Italian multicentre study. Eur. J. Clin. Pharmacol. 72, 117–123 (2016).
Costa, H., Costa, T. X., Martins, R. R. & Oliveira, A. G. Use of off-label and unlicensed medicines in neonatal intensive care. PLoS ONE 13, e0204427 (2018).
Acknowledgements
The research activities of A.S. are supported by the Clinical Research and Education Council of the University Hospitals Leuven.
Funding
No funding was used for the development of this paper.
Author information
Authors and Affiliations
Consortia
Contributions
Concept and design: L.M., G.R., S.B., R.B.F., S.Ü., H.K., G.C., F.G., A.B., E.M.D., K.A., S.H.P.S., A.S. Acquisition of data: L.M., G.R., S.B., R.B.F., S.Ü., G.C., F.G., K.A., S.H.P.S., A.S. Analysis and interpretation of data: L.M., G.R., S.B., R.B.F., S.Ü., H.K., G.C., F.G., A.B., E.M.D., K.A., S.H.P.S., A.S. Drafting the article: L.M., G.R., R.B.F., G.C., F.G., K.A., S.H.P.S., A.S. Critical revision of the article: L.M., G.R., S.B., R.B.F., S.Ü., H.K., G.C., F.G., A.B., E.M.D., K.A., S.H.P.S., A.S. Final approval of the version to be published: L.M., G.R., S.B., R.B.F., S.Ü., H.K., G.C., F.G., A.B., E.M.D., K.A., S.H.P.S., A.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
No patient consent was applicable for this paper.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mahoney, L., Raffaeli, G., Beken, S. et al. Grading the level of evidence of neonatal pharmacotherapy: midazolam and phenobarbital as examples. Pediatr Res 95, 75–83 (2024). https://doi.org/10.1038/s41390-023-02779-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02779-9