Abstract
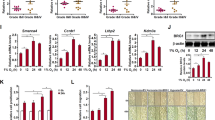
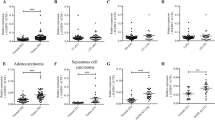
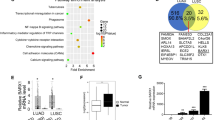
Lung adenocarcinoma is a malignant tumor with high morbidity and mortality. ZBTB16 plays a double role in various tumors; however, the potential mechanism of ZBTB16 in the pathophysiology of lung adenocarcinoma has yet to be elucidated. We herein observed a decreased expression of ZBTB16 mRNA and protein in lung adenocarcinoma and a significantly increased DNA methylation level of ZBTB16 in patients with lung adenocarcinoma. Analysis of public databases and patients’ clinical data indicated a close association between ZBTB16 and patient survival. Ectopic expression of ZBTB16 in lung adenocarcinoma cells significantly inhibited cell proliferation, invasion, and migration. It also induced cell cycle arrest in the S phase. Meanwhile, mitotic catastrophe was induced, and DNA damage and apoptosis occurred. In line with these findings, the overexpression of ZBTB16 in xenograft mice resulted in the inhibition of tumor growth. Comprehensive analysis showed that WDHD1 was a potential target for ZBTB16. The overexpression of both isoforms of WDHD1 significantly reversed the ZBTB16-mediated inhibition of lung adenocarcinoma proliferation and cell cycle. These studies suggest that ZBTB16 impedes the progression of lung adenocarcinoma by interfering with WDHD1 transcription, making it a potential novel therapeutic target in the management of lung adenocarcinoma.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The RNA-seq and ChIP-seq data generated in this study were deposited in NCBI’s Gene Expression Omnibus and were accessible through GEO Series accession numbers GSE263036 and GSE263037.
References
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48.
Relli V, Trerotola M, Guerra E, Alberti S. Abandoning the notion of non-small cell lung cancer. Trends Mol Med. 2019;25:585–94.
Wang M, Herbst RS, Boshoff C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat Med. 2021;27:1345–56.
Liu TM, Lee EH, Lim B, Shyh-Chang N. Concise review: Balancing stem cell self-renewal and differentiation with PLZF. Stem Cells. 2016;34:277–87.
Barna M, Merghoub T, Costoya JA, Ruggero D, Branford M, Bergia A, et al. Plzf mediates transcriptional repression of HoxD gene expression through chromatin remodeling. Dev Cell. 2002;3:499–510.
He J, Wu M, Xiong L, Gong Y, Yu R, Peng W, et al. BTB/POZ zinc finger protein ZBTB16 inhibits breast cancer proliferation and metastasis through upregulating ZBTB28 and antagonizing BCL6/ZBTB27. Clin Epigenetics. 2020;12:82.
Zhang T, Dong K, Liang W, Xu D, Xia H, Geng J, et al. G-protein-coupled receptors regulate autophagy by ZBTB16-mediated ubiquitination and proteasomal degradation of Atg14L. Elife. 2015;4:e06734.
Suliman BA, Xu D, Williams BR. The promyelocytic leukemia zinc finger protein: two decades of molecular oncology. Front Oncol. 2012;2:74.
Felicetti F, Bottero L, Felli N, Mattia G, Labbaye C, Alvino E, et al. Role of PLZF in melanoma progression. Oncogene. 2004;23:4567–76.
Hui AW, Lau HW, Cao CY, Zhou JW, Lai PB, Tsui SK. Downregulation of PLZF in human hepatocellular carcinoma and its clinical significance. Oncol Rep. 2015;33:397–402.
Vincent A, Omura N, Hong SM, Jaffe A, Eshleman J, Goggins M. Genome-wide analysis of promoter methylation associated with gene expression profile in pancreatic adenocarcinoma. Clin Cancer Res. 2011;17:4341–54.
Jin Y, Nenseth HZ, Saatcioglu F. Role of PLZF as a tumor suppressor in prostate cancer. Oncotarget. 2017;8:71317–24.
Choi WI, Kim MY, Jeon BN, Koh DI, Yun CO, Li Y, et al. Role of promyelocytic leukemia zinc finger (PLZF) in cell proliferation and cyclin-dependent kinase inhibitor 1A (p21WAF/CDKN1A) gene repression. J Biol Chem. 2014;289:18625–40.
Shen H, Zhan M, Zhang Y, Huang S, Xu S, Huang X, et al. PLZF inhibits proliferation and metastasis of gallbladder cancer by regulating IFIT2. Cell Death Dis. 2018;9:71.
Xiao GQ, Li F, Findeis-Hosey J, Hyrien O, Unger PD, Xiao L, et al. Down-regulation of cytoplasmic PLZF correlates with high tumor grade and tumor aggression in non-small cell lung carcinoma. Hum Pathol. 2015;46:1607–15.
Xian Q, Zhu D. The involvement of WDHD1 in the occurrence of esophageal cancer as a downstream target of PI3K/AKT pathway. J Oncol. 2022;2022:5871188.
Li Y, Xiao H, de Renty C, Jaramillo-Lambert A, Han Z, DePamphilis ML, et al. The involvement of acidic nucleoplasmic DNA-binding protein (And-1) in the regulation of prereplicative complex (pre-RC) assembly in human cells. J Biol Chem. 2012;287:42469–79.
Zhu W, Ukomadu C, Jha S, Senga T, Dhar SK, Wohlschlegel JA, et al. Mcm10 and And-1/CTF4 recruit DNA polymerase alpha to chromatin for initiation of DNA replication. Genes Dev. 2007;21:2288–99.
Yoshizawa-Sugata N, Masai H. Roles of human AND-1 in chromosome transactions in S phase. J Biol Chem. 2009;284:20718–28.
Sato N, Koinuma J, Fujita M, Hosokawa M, Ito T, Tsuchiya E, et al. Activation of WD repeat and high-mobility group box DNA binding protein 1 in pulmonary and esophageal carcinogenesis. Clin Cancer Res. 2010;16:226–39.
Hsieh CL, Lin CL, Liu H, Chang YJ, Shih CJ, Zhong CZ, et al. WDHD1 modulates the post-transcriptional step of the centromeric silencing pathway. Nucleic Acids Res. 2011;39:4048–62.
Gong L, Xiao M, He D, Hu Y, Zhu Y, Xiang L, et al. WDHD1 leads to cisplatin resistance by promoting MAPRE2 ubiquitination in lung adenocarcinoma. Front Oncol. 2020;10:461.
Gou W, Yu X, Wu S, Wu H, Chang H, Chen L, et al. Targeted inhibition of acidic nucleoplasmic DNA-binding protein 1 enhances radiosensitivity of non-small cell lung cancer. Cancer Lett. 2022;530:100–9.
Christman JK. 5-Azacytidine and 5-aza-2’-deoxycytidine as inhibitors of DNA methylation: mechanistic studies and their implications for cancer therapy. Oncogene. 2002;21:5483–95.
Vitale I, Galluzzi L, Castedo M, Kroemer G. Mitotic catastrophe: a mechanism for avoiding genomic instability. Nat Rev Mol Cell Biol. 2011;12:385–92.
Chiou L-W, Chan C-H, Jhuang Y-L, Yang C-Y, Jeng Y-M. DNA replication stress and mitotic catastrophe mediate sotorasib addiction in KRASG12C-mutant cancer. J Biomed Sci. 2023;30:50.
Yeyati PL, Shaknovich R, Boterashvili S, Li J, Ball HJ, Waxman S, et al. Leukemia translocation protein PLZF inhibits cell growth and expression of cyclin A. Oncogene. 1999;18:925–34.
Xiao GQ, Unger P, Yang Q, Kinoshita Y, Singh K, McMahon L, et al. Loss of PLZF expression in prostate cancer by immunohistochemistry correlates with tumor aggressiveness and metastasis. PLoS ONE. 2015;10:e0121318.
Wang X, Wang L, Guo S, Bao Y, Ma Y, Yan F, et al. Hypermethylation reduces expression of tumor-suppressor PLZF and regulates proliferation and apoptosis in non-small-cell lung cancers. FASEB J. 2013;27:4194–203.
Wischhof L, Scifo E, Ehninger D, Bano D. AIFM1 beyond cell death: an overview of this OXPHOS-inducing factor in mitochondrial diseases. EBioMedicine. 2022;83:104231.
Cowell IG, Casement JW, Austin CA. To break or not to break: the role of TOP2B in transcription. Int J Mol Sci. 2023;24:14806.
Nyamao RM, Wu J, Yu L, Xiao X, Zhang FM. Roles of DDX5 in the tumorigenesis, proliferation, differentiation, metastasis and pathway regulation of human malignancies. Biochim Biophys Acta Rev Cancer. 2019;1871:85–98.
Legrand JMD, Chan A-L, La HM, Rossello FJ, Änkö M-L, Fuller-Pace FV, et al. DDX5 plays essential transcriptional and post-transcriptional roles in the maintenance and function of spermatogonia. Nat Commun. 2019;10:2278.
Jaramillo-Lambert A, Hao J, Xiao H, Li Y, Han Z, Zhu W. Acidic nucleoplasmic DNA-binding protein (And-1) controls chromosome congression by regulating the assembly of centromere protein A (CENP-A) at centromeres. J Biol Chem. 2013;288:1480–8.
Sun S, Wang K, Guo D, Zheng H, Liu Y, Shen H, et al. Identification of the key DNA damage response genes for predicting immunotherapy and chemotherapy efficacy in lung adenocarcinoma based on bulk, single-cell RNA sequencing, and spatial transcriptomics. Comput Biol Med. 2024;171:108078.
Acknowledgements
We thank all TCGA, GTEx, GEO database researchers and patients involved in the article, for their willingness to share relevant data and their contributions to medical progress. The graphical abstract is drawn by FigDraw.
Funding
This work is supported by National Natural Science Foundation of China (82102700) and Natural Science Foundation of Shandong Province (ZR2021MH192).
Author information
Authors and Affiliations
Contributions
Jiajun Du, Kai Wang and Deyu Guo contributed to the conception and supervision of this research. Co-authors Kai Wang, Deyu Guo, Tao Yan and Yadong Wang cultured cells, collected research materials and designed the experiment. Kai Wang, Deyu Guo, Shijie Sun, Yadong Wang, Haotian Zheng and Guanghui Wang collected patient information and conducted follow-up. Kai Wang, Deyu Guo, Tao Yan and Shijie Sun writing, review and editing this article. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, K., Guo, D., Yan, T. et al. ZBTB16 inhibits DNA replication and induces cell cycle arrest by targeting WDHD1 transcription in lung adenocarcinoma. Oncogene (2024). https://doi.org/10.1038/s41388-024-03041-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41388-024-03041-0