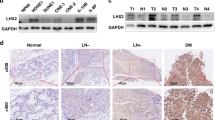
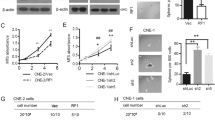
Abstract
Transcription factors (TFs) engage in various cellular essential processes including differentiation, growth and migration. However, the master TF involved in distant metastasis of nasopharyngeal carcinoma (NPC) remains largely unclear. Here we show that KLF5 regulates actin remodeling to enhance NPC metastasis. We analyzed the msVIPER algorithm-generated transcriptional regulatory networks and identified KLF5 as a master TF of metastatic NPC linked to poor clinical outcomes. KLF5 regulates actin remodeling and lamellipodia formation to promote the metastasis of NPC cells in vitro and in vivo. Mechanistically, KLF5 preferentially occupies distal enhancer regions of ACTN4 to activate its transcription, whereby decoding the informative DNA sequences. ACTN4, extensively localized within actin cytoskeleton, facilitates dense and branched actin networks and lamellipodia formation at the cell leading edge, empowering cells to migrate faster. Collectively, our findings reveal that KLF5 controls robust transcription program of ACTN4 to modulate actin remodeling and augment cell motility which enhances NPC metastasis, and provide new potential biomarkers and therapeutic interventions for NPC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article and its Supplementary Information Files.
References
Gerstberger S, Jiang Q, Ganesh K. Metastasis. Cell. 2023;186:1564–79.
Massagué J, Ganesh K. Metastasis-initiating cells and ecosystems. Cancer Discov. 2021;11:971–94.
Follain G, Herrmann D, Harlepp S, Hyenne V, Osmani N, Warren SC, et al. Fluids and their mechanics in tumour transit: shaping metastasis. Nat Rev Cancer. 2020;20:107–24.
SenGupta S, Parent CA, Bear JE. The principles of directed cell migration. Nat Rev Mol Cell Biol. 2021;22:529–47.
Fu A, Yao B, Dong T, Chen Y, Yao J, Liu Y, et al. Tumor-resident intracellular microbiota promotes metastatic colonization in breast cancer. Cell. 2022;185:1356–1372.e26.
Bera K, Kiepas A, Godet I, Li Y, Mehta P, Ifemembi B, et al. Extracellular fluid viscosity enhances cell migration and cancer dissemination. Nature. 2022;611:365–73.
Hakala M, Wioland H, Tolonen M, Kotila T, Jegou A, Romet-Lemonne G, et al. Twinfilin uncaps filament barbed ends to promote turnover of lamellipodial actin networks. Nat Cell Biol. 2021;23:147–59.
Banerjee T, Biswas D, Pal DS, Miao Y, Iglesias PA, Devreotes PN. Spatiotemporal dynamics of membrane surface charge regulates cell polarity and migration. Nat Cell Biol. 2022;24:1499–515.
Reversat A, Gaertner F, Merrin J, Stopp J, Tasciyan S, Aguilera J, et al. Cellular locomotion using environmental topography. Nature. 2020;582:582–5.
Mehidi A, Kage F, Karatas Z, Cercy M, Schaks M, Polesskaya A, et al. Forces generated by lamellipodial actin filament elongation regulate the WAVE complex during cell migration. Nat Cell Biol. 2021;23:1148–62.
De Belly H, Yan S, Borja da Rocha H, Ichbiah S, Town JP, Zager PJ, et al. Cell protrusions and contractions generate long-range membrane tension propagation. Cell. 2023;186:3049–61.e15.
Bisaria A, Hayer A, Garbett D, Cohen D, Meyer T. Membrane-proximal F-actin restricts local membrane protrusions and directs cell migration. Science. 2020;368:1205–10.
Lappalainen P, Kotila T, Jégou A, Romet-Lemonne G. Biochemical and mechanical regulation of actin dynamics. Nat Rev Mol Cell Biol. 2022;23:836–52.
Liu X, Nie L, Zhang Y, Yan Y, Wang C, Colic M, et al. Actin cytoskeleton vulnerability to disulfide stress mediates disulfidptosis. Nat Cell Biol. 2023;25:404–14.
Zou H, Poore B, Brown EE, Qian J, Xie B, Asimakidou E, et al. A neurodevelopmental epigenetic programme mediated by SMARCD3-DAB1-Reelin signalling is hijacked to promote medulloblastoma metastasis. Nat Cell Biol. 2023;25:493–507.
Tetreault M-P, Yang Y, Katz JP. Krüppel-like factors in cancer. Nat Rev Cancer. 2013;13:701–13.
Chen Y, Lüttmann FF, Schoger E, Schöler HR, Zelarayán LC, Kim K-P, et al. Reversible reprogramming of cardiomyocytes to a fetal state drives heart regeneration in mice. Science. 2021;373:1537–40.
Blacher E, Tsai C, Litichevskiy L, Shipony Z, Iweka CA, Schneider KM, et al. Aging disrupts circadian gene regulation and function in macrophages. Nat Immunol. 2022;23:229–6.
Wang Z, Yang L, Wu P, Li X, Tang Y, Ou X, et al. The circROBO1/KLF5/FUS feedback loop regulates the liver metastasis of breast cancer by inhibiting the selective autophagy of afadin. Mol Cancer. 2022;21:9.
Zeng L, Zhu Y, Moreno CS, Wan Y. New insights into KLFs and SOXs in cancer pathogenesis, stemness, and therapy. Semin Cancer Biol. 2023;90:29–44.
Liu P, Wang Z, Ou X, Wu P, Zhang Y, Wu S, et al. The FUS/circEZH2/KLF5/ feedback loop contributes to CXCR4-induced liver metastasis of breast cancer by enhancing epithelial-mesenchymal transition. Mol Cancer. 2022;21:198.
Zhang B, Li Y, Wu Q, Xie L, Barwick B, Fu C, et al. Acetylation of KLF5 maintains EMT and tumorigenicity to cause chemoresistant bone metastasis in prostate cancer. Nat Commun. 2021;12:1714.
Wu Y, Chen S, Shao Y, Su Y, Li Q, Wu J, et al. KLF5 promotes tumor progression and parp inhibitor resistance in ovarian cancer. Adv Sci. 2023;10:e2304638.
Di Giammartino DC, Kloetgen A, Polyzos A, Liu Y, Kim D, Murphy D, et al. KLF4 is involved in the organization and regulation of pluripotency-associated three-dimensional enhancer networks. Nat Cell Biol. 2019;21:1179–90.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global cancer observatory: cancer today. International Agency for Research on Cancer, Lyon, France. 2020. https://gco.iarc.fr/today. Accessed 2 Aug 2023.
Zhang Y, Chen L, Hu G-Q, Zhang N, Zhu X-D, Yang K-Y, et al. Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N Engl J Med. 2019;381:1124–35.
Yang Y, Pan J, Wang H, Zhao Y, Qu S, Chen N, et al. Tislelizumab plus chemotherapy as first-line treatment for recurrent or metastatic nasopharyngeal cancer: a multicenter phase 3 trial (RATIONALE-309). Cancer Cell. 2023;41:1061–1072.e4.
Lachmann A, Giorgi FM, Lopez G, Califano A. ARACNe-AP: gene network reverse engineering through adaptive partitioning inference of mutual information. Bioinformatics. 2016;32:2233–5.
Lambert SA, Jolma A, Campitelli LF, Das PK, Yin Y, Albu M, et al. The human transcription factors. Cell. 2018;172:650–65.
Alvarez MJ, Shen Y, Giorgi FM, Lachmann A, Ding BB, Ye BH, et al. Functional characterization of somatic mutations in cancer using network-based inference of protein activity. Nat Genet. 2016;48:838–47.
He P, Yang JW, Yang VW, Bialkowska AB. Krüppel-like Factor 5, increased in pancreatic ductal adenocarcinoma, promotes proliferation, acinar-to-ductal metaplasia, pancreatic intraepithelial neoplasia, and tumor growth in mice. Gastroenterology. 2018;154:1494–1508.e13.
Kim J, Wang C, de Sabando AR, Cole HL, Huang TJ, Yang J, et al. The novel small-molecule SR18662 efficiently inhibits the growth of colorectal cancer in vitro and in vivo. Mol Cancer Ther. 2019;18:1973–84.
Mueller J, Szep G, Nemethova M, de Vries I, Lieber AD, Winkler C, et al. Load adaptation of lamellipodial actin networks. Cell. 2017;171:188–200.e16.
Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, et al. Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet. 2000;24:251–6.
Wang M-C, Chang Y-H, Wu C-C, Tyan Y-C, Chang H-C, Goan Y-G, et al. Alpha-actinin 4 is associated with cancer cell motility and is a potential biomarker in non-small cell lung cancer. J Thorac Oncol. 2015;10:286–301.
Huang Z, Zhou J-K, Wang K, Chen H, Qin S, Liu J, et al. PDLIM1 inhibits tumor metastasis through activating hippo signaling in hepatocellular carcinoma. Hepatology. 2020;71:1643–59.
Feng D, Kumar M, Muntel J, Gurley SB, Birrane G, Stillman IE, et al. Phosphorylation of ACTN4 leads to podocyte vulnerability and proteinuric glomerulosclerosis. J Am Soc Nephrol. 2020;31:1479–95.
Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJE. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10:845–58.
Yan Y, Tao H, He J, Huang S-Y. The HDOCK server for integrated protein-protein docking. Nat Protoc. 2020;15:1829–52.
DelRosso, Tycko N, Suzuki J, Andrews P, Aradhana C, Mukund A, et al. Large-scale mapping and mutagenesis of human transcriptional effector domains. Nature. 2023;616:365–72.
Kim K, Jang I, Kim M, Choi J, Kim M-S, Lee B, et al. 3DIV update for 2021: a comprehensive resource of 3D genome and 3D cancer genome. Nucleic Acids Res. 2021;49:D38–D46.
Huang Q, Liu M, Zhang D, Lin B-B, Fu X, Zhang Z, et al. Nitazoxanide inhibits acetylated KLF5-induced bone metastasis by modulating KLF5 function in prostate cancer. BMC Med. 2023;21:68.
Wei Z, Gao F, Kim S, Yang H, Lyu J, An W, et al. Klf4 organizes long-range chromosomal interactions with the oct4 locus in reprogramming and pluripotency. Cell Stem Cell. 2013;13:36–47.
Chronis C, Fiziev P, Papp B, Butz S, Bonora G, Sabri S, et al. Cooperative binding of transcription factors orchestrates reprogramming. Cell. 2017;168:442–59.e20.
Michael AK, Thomä NH. Reading the chromatinized genome. Cell. 2021;184:3599–611.
Larson ED, Marsh AJ, Harrison MM. Pioneering the developmental frontier. Mol Cell. 2021;81:1640–50.
Jiang J, Chan Y-S, Loh Y-H, Cai J, Tong G-Q, Lim C-A, et al. A core Klf circuitry regulates self-renewal of embryonic stem cells. Nat Cell Biol. 2008;10:353–60.
Soufi A, Donahue G, Zaret KS. Facilitators and impediments of the pluripotency reprogramming factors’ initial engagement with the genome. Cell. 2012;151:994–1004.
Dos Santos M, Shah AM, Zhang Y, Bezprozvannaya S, Chen K, Xu L, et al. Opposing gene regulatory programs governing myofiber development and maturation revealed at single nucleus resolution. Nat Commun. 2023;14:4333.
Liu Y, Guo B, Aguilera-Jimenez E, Chu VS, Zhou J, Wu Z, et al. Chromatin looping shapes KLF5-dependent transcriptional programs in human epithelial cancers. Cancer Res. 2020;80:5464–77.
Jiang YY, Jiang Y, Li CQ, Zhang Y, Dakle P, Kaur H, et al. TP63, SOX2, and KLF5 establish a core regulatory circuitry that controls epigenetic and transcription patterns in esophageal squamous cell carcinoma cell lines. Gastroenterology. 2020;159:1311–27.
Yolland L, Burki M, Marcotti S, Luchici A, Kenny FN, Davis JR, et al. Persistent and polarized global actin flow is essential for directionality during cell migration. Nat Cell Biol. 2019;21:1370–81.
Schoenfelder S, Fraser P. Long-range enhancer-promoter contacts in gene expression control. Nat Rev Genet. 2019;20:437–55.
Winick-Ng W, Kukalev A, Harabula I, Zea-Redondo L, Szabó D, Meijer M, et al. Cell-type specialization is encoded by specific chromatin topologies. Nature. 2021;599:684–91.
Goel VY, Huseyin MK, Hansen AS. Region capture micro-C reveals coalescence of enhancers and promoters into nested microcompartments. Nat Genet. 2023;55:1048–56.
Steeg PS. Targeting metastasis. Nat Rev Cancer. 2016;16:201–18.
Weiss F, Lauffenburger D, Friedl P. Towards targeting of shared mechanisms of cancer metastasis and therapy resistance. Nat Rev Cancer. 2022;22:157–73.
Bushweller JH. Targeting transcription factors in cancer - from undruggable to reality. Nat Rev Cancer. 2019;19:611–24.
Morita K, Suzuki K, Maeda S, Matsuo A, Mitsuda Y, Tokushige C, et al. Genetic regulation of the RUNX transcription factor family has antitumor effects. J Clin Invest. 2017;127:2815–28.
Békés M, Langley DR, Crews CM. PROTAC targeted protein degraders: the past is prologue. Nat Rev Drug Discov. 2022;21:181–200.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (92259202, 82273401, 82103579, 82102875), the Guangdong Basic and Applied Basic Research Foundation (2019A1515011863, 2019A1515012045, 2023B1515020014), the Health & Medical Collaborative Innovation Project of Guangzhou City, China (202201011261), the Special Support Program of Sun Yat-sen University Cancer Center (16zxtzlc06), the Cancer Innovative Research Program of Sun Yat-sen University Cancer Center (CIRP-SYSUCC-0010), the Young Talents Program of Sun Yat-sen University Cancer Center (YTP-SYSUCC-0069) and the Sanming Project of Medicine in Shenzhen(SZSM202211017).
Author information
Authors and Affiliations
Contributions
GZ and DW designed and supervised the study. GZ, DW and ZY conducted the literature search. ZY, YP, YW and PY performed the experiments. JL, ZY and XX performed the bioinformatic analysis. ZH developed the animal models. TQ, ZY, PS and YP extracted and analyzed data. GZ, ZY, DW and YS were responsible for interpreting results. ZY, YW, DW and GZ contributed to the figures and tables. ZY and DW wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Z., Peng, Y., Wang, Y. et al. KLF5 regulates actin remodeling to enhance the metastasis of nasopharyngeal carcinoma. Oncogene (2024). https://doi.org/10.1038/s41388-024-03033-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41388-024-03033-0