Abstract
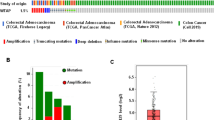
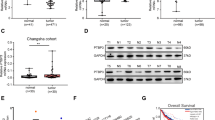
N6-methyladenosine (m6A), the most abundant mRNA modification in mammals, is involved in the metabolism of mRNA. KIAA1429 is regarded as the largest m6A methyltransferase and plays an important role in m6A modification. However, the prognostic value and function of KIAA1429 in colorectal cancer (CRC) are unclear. Quantitative real-time PCR and immunohistochemical assays were performed to evaluate the expression of KIAA1429 in CRC tissues. Kaplan–Meier survival curves and log-rank tests were used to assess the association between KIAA1429 expression and the prognosis of patients with CRC. CCK-8 assays, colony formation assays, cell cycle assays, and xenograft experiments were performed to investigate the effect of KIAA1429 on cell proliferation. RNA immunoprecipitation, methylated RNA immunoprecipitation assays, and RNA stability assays were conducted to explore the underlying mechanism. KIAA1429 was significantly upregulated in CRC tissues compared with adjacent normal tissues. Patients with higher expression of KIAA1429 had shorter overall survival than those with lower expression. Functionally, KIAA1429 promoted CRC cell proliferation in vitro and in vivo. Mechanistically, KIAA1429 negatively regulated the expression of WEE1 by decreasing its stability in an m6A-independent manner by binding to the third segment in the 3′-UTR of WEE1 mRNA. Moreover, butyrate decreased the expression of KIAA1429 by downregulating the level of the transcription factor NFκB1. Our findings indicated that KIAA1429 plays an oncogenic role in CRC cells by inhibiting the expression of WEE1 in an m6A-independent manner and is associated with poor survival in CRC patients. These results suggested that KIAA1429 might be a potential prognostic marker for CRC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49.
van der Geest LGM, Lam-Boer JT, Koopman M, Verhoef C, Elferink MAG, de Wilt JHW. Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin Exp Metastasis. 2015;32:457–65.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34.
Pan Y, Ma P, Liu Y, Li W, Shu Y. Multiple functions of m(6)A RNA methylation in cancer. J Hematol Oncol. 2018;11:48.
Yue B, Song C, Yang L, Cui R, Cheng X, Zhang Z, et al. METTL3-mediated N6-methyladenosine modification is critical for epithelial-mesenchymal transition and metastasis of gastric cancer. Mol Cancer. 2019;18:142.
Chen Y, Peng C, Chen J, Chen D, Yang B, He B, et al. WTAP facilitates progression of hepatocellular carcinoma via m6A-HuR-dependent epigenetic silencing of ETS1. Mol Cancer. 2019;18:127.
Cai X, Wang X, Cao C, Gao Y, Zhang S, Yang Z, et al. HBXIP-elevated methyltransferase METTL3 promotes the progression of breast cancer via inhibiting tumor suppressor let-7g. Cancer Lett. 2018;415:11–9.
Peng W, Li J, Chen R, Gu Q, Yang P, Qian W, et al. Upregulated METTL3 promotes metastasis of colorectal Cancer via miR-1246/SPRED2/MAPK signaling pathway. J Exp Clin Cancer Res. 2019;38:393.
Bai Y, Yang C, Wu R, Huang L, Song S, Li W, et al. YTHDF1 Regulates Tumorigenicity and Cancer Stem Cell-Like Activity in Human Colorectal Carcinoma. Int J Cancer. 2019;9:332.
Chen X, Xu M, Xu X, Zeng K, Liu X, Pan B, et al. METTL14-mediated N6-methyladenosine modification of SOX4 mRNA inhibits tumor metastasis in colorectal cancer. Mol Cancer. 2020;19:106.
Li T, Hu PS, Zuo Z, Lin JF, Li X, Wu QN, et al. METTL3 facilitates tumor progression via m(6)A-IGF2BP2-Depend mechanism colorectal carcinoma. Mol Cancer. 2019;18:112.
Ni W, Yao S, Zhou Y, Liu Y, Huang P, Zhou A, et al. Long noncoding RNA GAS5 inhibits progression of colorectal cancer by interacting with and triggering YAP phosphorylation and degradation and is negatively regulated by the m(6)A reader YTHDF3. Mol Cancer. 2019;18:143.
Nishizawa Y, Konno M, Asai A, Koseki J, Kawamoto K, Miyoshi N, et al. Oncogene c-Myc promotes epitranscriptome m(6)A reader YTHDF1 expression in colorectal cancer. Oncotarget. 2018;9:7476–86.
Wang Y, Lu JH, Wu QN, Jin Y, Wang DS, Chen YX, et al. LncRNA LINRIS stabilizes IGF2BP2 and promotes the aerobic glycolysis in colorectal cancer. Mol Cancer. 2019;18:174.
Yang P, Wang Q, Liu A, Zhu J, Feng J. ALKBH5 Holds Prognostic Values and Inhibits the Metastasis of Colon Cancer. Pathol Oncol Res. 2020;26:1615–23.
Chen X, Xu M, Xu X, Zeng K, Liu X, Sun L, et al. METTL14 Suppresses CRC Progression via Regulating N6-Methyladenosine-Dependent Primary miR-375 Processing. Mol Ther. 2020;28:599–612.
Yue Y, Liu J, Cui X, Cao J, Luo G, Zhang Z, et al. VIRMA mediates preferential m(6)A mRNA methylation in 3’UTR and near stop codon and associates with alternative polyadenylation. Cell Disco. 2018;4:10.
Schwartz S, Mumbach MR, Jovanovic M, Wang T, Maciag K, Bushkin GG, et al. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5’ sites. Cell Rep. 2014;8:284–96.
Cheng X, Li M, Rao X, Zhang W, Li X, Wang L, et al. KIAA1429 regulates the migration and invasion of hepatocellular carcinoma by altering m6A modification of ID2 mRNA. Onco Targets Ther. 2019;12:3421–8.
Lan T, Li H, Zhang D, Xu L, Liu H, Hao X, et al. KIAA1429 contributes to liver cancer progression through N6-methyladenosine-dependent post-transcriptional modification of GATA3. Mol Cancer. 2019;18:186.
Barros-Silva D, Lobo J, Guimaraes-Teixeira C, Carneiro I, Oliveira J, Martens-Uzunova ES, et al. VIRMA-Dependent N6-Methyladenosine Modifications Regulate the Expression of Long Non-Coding RNAs CCAT1 and CCAT2 in Prostate Cancer. Cancers (Basel). 2020;12.771.
Qian JY, Gao J, Sun X, Cao MD, Shi L, Xia TS, et al. KIAA1429 acts as an oncogenic factor in breast cancer by regulating CDK1 in an N6-methyladenosine-independent manner. Oncogene. 2019;38:6123–41.
Miao R, Dai CC, Mei L, Xu J, Sun SW, Xing YL, et al. KIAA1429 regulates cell proliferation by targeting c-Jun messenger RNA directly in gastric cancer. J Cell Physiol. 2020;235:7420–32.
Zhu W, Wang JZ, Wei JF, Lu C. Role of m6A methyltransferase component VIRMA in multiple human cancers (Review). Cancer Cell Int. 2021;21:172.
Wang X, Fu X, Zhang J, Xiong C, Zhang S, Lv Y. Identification and validation of m(6)A RNA methylation regulators with clinical prognostic value in Papillary thyroid cancer. Cancer Cell Int. 2020;20:203.
Zhu W, Si Y, Xu J, Lin Y, Wang JZ, Cao M, et al. Methyltransferase like 3 promotes colorectal cancer proliferation by stabilizing CCNE1 mRNA in an m6A-dependent manner. J Cell Mol Med. 2020;24:3521–33.
Goncalves P, Martel F. Butyrate and colorectal cancer: the role of butyrate transport. Curr Drug Metab. 2013;14:994–1008.
Liu Y, Upadhyaya B, Fardin-Kia AR, Juenemann RM, Dey M. Dietary resistant starch type 4-derived butyrate attenuates nuclear factor-kappa-B1 through modulation of histone H3 trimethylation at lysine 27. Food Funct. 2016;7:3772–81.
Li Y, Xiao J, Bai J, Tian Y, Qu Y, Chen X, et al. Molecular characterization and clinical relevance of m(6)A regulators across 33 cancer types. Mol Cancer. 2019;18:137.
Russell P, Nurse P. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell. 1987;49:559–67.
Watanabe N, Broome M, Hunter T. Regulation of the human WEE1Hu CDK tyrosine 15-kinase during the cell cycle. Embo J. 1995;14:1878–91.
Domínguez-Kelly R, Martín Y, Koundrioukoff S, Tanenbaum ME, Smits VA, Medema RH, et al. Wee1 controls genomic stability during replication by regulating the Mus81-Eme1 endonuclease. J Cell Biol. 2011;194:567–79.
Backert S, Gelos M, Kobalz U, Hanski ML, Bohm C, Mann B, et al. Differential gene expression in colon carcinoma cells and tissues detected with a cDNA array. Int J Cancer. 1999;82:868–74.
Ge XC, Wu F, Li WT, Zhu XJ, Liu JW, Wang BL. Upregulation of WEE1 is a potential prognostic biomarker for patients with colorectal cancer. Oncol Lett. 2017;13:4341–8.
Wang X, Li Y, Chen W, Shi H, Eren AM, Morozov A, et al. Transcriptome-wide reprogramming of N6-methyladenosine modification by the mouse microbiome. Cell Res. 2018;29:167–70.
Wu X, Wu Y, He L, Wu L, Wang X, Liu Z. Effects of the intestinal microbial metabolite butyrate on the development of colorectal cancer. J Cancer. 2018;9:2510–7.
Encarnacao JC, Abrantes AM, Pires AS, Botelho MF. Revisit dietary fiber on colorectal cancer: butyrate and its role on prevention and treatment. Cancer Metastasis Rev. 2015;34:465–78.
Wang JJ, Wei ZK, Zhang X, Wang YN, Fu YH, Yang ZT. Butyrate protects against disruption of the blood-milk barrier and moderates inflammatory responses in a model of mastitis induced by lipopolysaccharide. Br J Pharm. 2017;174:3811–22.
Patel M, Horgan PG, McMillan DC, Edwards J. NF-kappaB pathways in the development and progression of colorectal cancer. Transl Res. 2018;197:43–56.
Acknowledgements
This study was supported by grants from Natural Science Foundation of Jiangsu Province (BK20201495), Jiangsu Province’s Key Provincial Talents Program (ZDRCA2016089), and the National Natural Science Foundation of China (No. 82102981).
Author information
Authors and Affiliations
Contributions
LJZ, JFW, TC and LM conceptualized and supervised the research. LM, YL, SWS and JX designed and performed most experiments. YL, TY and LM performed animal experiments. LM, YL, SWS, JX and JFW were engaged in biostatistics and bioinformatics analysis. WLC, LHZ, YCG, YWW, TC and LJZ were responsible for patient recruitment, biospecimen and clinical data collection. LM, YL and JFW wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Ma, L., Lin, Y., Sun, SW. et al. KIAA1429 is a potential prognostic marker in colorectal cancer by promoting the proliferation via downregulating WEE1 expression in an m6A-independent manner. Oncogene 41, 692–703 (2022). https://doi.org/10.1038/s41388-021-02066-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-021-02066-z
This article is cited by
-
METTL3 Promotes OSCC Progression by Down-Regulating WEE1 in a m6A-YTHDF2-Dependent Manner
Molecular Biotechnology (2024)
-
CRISPR-Cas9 knockout screening identifies KIAA1429 as an essential gene in Ewing sarcoma
Journal of Experimental & Clinical Cancer Research (2023)
-
Aberrant RNA m6A modification in gastrointestinal malignancies: versatile regulators of cancer hallmarks and novel therapeutic opportunities
Cell Death & Disease (2023)
-
lncRNA POU6F2-AS1 Regulated by KIAA1429 Contributes to Colorectal Cancer Progression in an m6A Modification Manner
Molecular Biotechnology (2023)
-
Overexpression of VIRMA confers vulnerability to breast cancers via the m6A-dependent regulation of unfolded protein response
Cellular and Molecular Life Sciences (2023)