Abstract
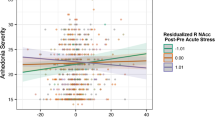
Elevated levels of systemic inflammation are associated with altered reward-related brain function in ventral striatal areas of the brain like the nucleus accumbens (NAcc). In adolescents, cross-sectional research indicates that exposure to early life stress (ELS) can moderate the relation between inflammation and neural activation, which may contribute to atypical reward function; however, no studies have tested whether this moderation by ELS of neuroimmune associations persists over time. Here, we conducted a cross-sectional analysis and the first exploratory longitudinal analysis testing whether cumulative severity of ELS moderates the association of systemic inflammation with reward-related processing in the NAcc in adolescents (n = 104; 58F/46M; M[SD] age = 16.00[1.45] years; range = 13.07–19.86 years). For the cross-sectional analysis, we modeled a statistical interaction between ELS and levels of C-reactive protein (CRP) predicting NAcc activation during the anticipation and outcome phases of a monetary reward task. We found that higher CRP was associated with blunted NAcc activation during the outcome of reward in youth who experienced higher levels of ELS (β = –0.31; p = 0.006). For the longitudinal analysis, we modeled an interaction between ELS and change in CRP predicting change in NAcc activation across 2 years. This analysis similarly showed that increasing CRP over time was associated with decreasing NAcc during reward outcomes in youth who experienced higher levels of ELS (β = –0.47; p = 0.022). Both findings support contemporary theoretical frameworks involving associations among inflammation, reward-related brain function, and ELS exposure, and suggest that experiencing ELS can have significant and enduring effects on neuroimmune function and adolescent neurodevelopment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25:1822–32.
Capuron L, Miller AH. Cytokines and psychopathology: lessons from interferon-α. Biol Psychiatry. 2004;56:819–24.
Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56.
Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002;25:154–9.
Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull. 2014;140:774–815.
Salvador AF, de Lima KA, Kipnis J. Neuromodulation by the immune system: a focus on cytokines. Nat Rev Immunol. 2021;21:526–41.
Felger JC, Treadway MT. Inflammation effects on motivation and motor activity: role of dopamine. Neuropsychopharmacology. 2017;42:216–41.
Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, et al. Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch Gen Psychiatry. 2012;69:1044–53.
Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry. 2010;68:748–54.
Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, et al. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatry. 2016;21:1358–65.
Eisenberger NI, Moieni M. Inflammation affects social experience: implications for mental health. World Psychiatry. 2020;19:109–10.
Inagaki TK, Muscatell KA, Irwin MR, Moieni M, Dutcher JM, Jevtic I, et al. The role of the ventral striatum in inflammatory-induced approach toward support figures. Brain Behav Immun. 2015;44:247–52.
Muscatell KA, Moieni M, Inagaki TK, Dutcher JM, Jevtic I, Breen EC, et al. Exposure to an inflammatory challenge enhances neural sensitivity to negative and positive social feedback. Brain Behav Immun. 2016;57:21–9.
Swartz JR, Carranza AF, Tully LM, Knodt AR, Jiang J, Irwin MR, et al. Associations between peripheral inflammation and resting state functional connectivity in adolescents. Brain Behav Immun. 2021;95:96–105.
Irwin MR, Eisenberger NI. Context-dependent effects of inflammation: reduced reward responding is not an invariant outcome of sickness. Neuropsychopharmacol. 2017;42:785–6.
McLaughlin KA, Greif Green J, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood adversities and first onset of psychiatric disorders in a National Sample of US adolescents. Arch Gen Psychiatry. 2012;69:1151–60.
Goldsmith DR, Bekhbat M, Mehta ND, Felger JC. Inflammation-related functional and structural dysconnectivity as a pathway to psychopathology. Biol Psychiatry. 2023;93:405–18.
Lumertz FS, Kestering-Ferreira E, Orso R, Creutzberg KC, Tractenberg SG, Stocchero BA, et al. Effects of early life stress on brain cytokines: a systematic review and meta-analysis of rodent studies. Neurosci Biobehav Rev. 2022;139:104746.
Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychological Bull. 2004;130:601–30.
Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 2007;21:901–12.
Slopen N, Kubzansky LD, McLaughlin KA, Koenen KC. Childhood adversity and inflammatory processes in youth: a prospective study. Psychoneuroendocrinology. 2013;38:188–200.
Danese A, Caspi A, Williams B, Ambler A, Sugden K, Mika J, et al. Biological embedding of stress through inflammation processes in childhood. Mol Psychiatry. 2011;16:244–6.
Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc. Natl Acad. Sci. USA. 2007;104:1319–24.
Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology. 2011;214:55–70.
Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-Ruth K, Pizzagalli DA. Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biol Psychiatry. 2009;66:206–13.
Novick AM, Levandowski ML, Laumann LE, Philip NS, Price LH, Tyrka AR. The effects of early life stress on reward processing. J Psychiatr Res. 2018;101:80–103.
Nusslock R, Miller GE. Early-life adversity and physical and emotional health across the lifespan: a neuroimmune network hypothesis. Biol Psychiatry. 2016;80:23–32.
Kuhlman KR, Cole SW, Irwin MR, Craske MG, Fuligni AJ, Bower JE. The role of early life adversity and inflammation in stress-induced change in reward and risk processes among adolescents. Brain Behav Immun. 2023;109:78–88.
Chat IK, Gepty AA, Kautz M, Mac Giollabhui N, Adogli ZV, Coe CL, et al. Residence in high-crime neighborhoods moderates the association between interleukin 6 and social and nonsocial reward brain responses. Biol Psychiatry Glob Open Sci. 2022;2:273–82.
Miller GE, White SF, Chen E, Nusslock R. Association of inflammatory activity with larger neural responses to threat and reward among children living in poverty. Am J Psychiatry. 2021;178:313–20.
Chiang JJ, Lam PH, Chen E, Miller GE. Psychological stress during childhood and adolescence and its association with inflammation across the lifespan: a critical review and meta-analysis. Psychol Bull. 2022;148:27–66.
Galván A. Adolescent brain development and contextual influences: a decade in review. J Res Adolescence. 2021;31:843–69.
Hanson JL, Williams AV, Bangasser DA, Peña CJ. Impact of early life stress on reward circuit function and regulation. Front Psychiatry. 2021;12:744690.
Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolescence. 1980;9:271–80.
Ribbe D. Psychometric review of traumatic event screening instrument forchildren (TESI-C). In: Stamm BH, editor. Measurement of Stress, Trauma, and Adaptation. Lutherville, MD: Sidran Press; 1996. pp. 386–387.
Rudolph KD, Hammen C, Burge D, Lindberg N, Herzberg D, Daley SE. Toward an interpersonal life-stress model of depression: the developmental context of stress generation. Dev Psychopathol. 2000;12:215–34.
King LS, Colich NL, LeMoult J, Humphreys KL, Ordaz SJ, Price AN, et al. The impact of the severity of early life stress on diurnal cortisol: the role of puberty. Psychoneuroendocrinology. 2017;77:68–74.
King LS, Graber MG, Colich NL, Gotlib IH. Associations of waking cortisol with DHEA and testosterone across the pubertal transition: effects of threat-related early life stress. Psychoneuroendocrinology. 2020;115:104651.
Ho TC, Kulla A, Teresi GI, Sisk LM, Rosenberg-Hasson Y, Maecker HT, et al. Inflammatory cytokines and callosal white matter microstructure in adolescents. Brain Behav Immun. 2022;100:321–31.
Yuan JP, Ho TC, Coury SM, Chahal R, Colich NL, Gotlib IH. Early life stress, systemic inflammation, and neural correlates of implicit emotion regulation in adolescents. Brain Behav Immun. 2022;105:169–79.
Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159–RC159.
Gotlib IH, Hamilton JP, Cooney RE, Singh MK, Henry ML, Joormann J. Neural processing of reward and loss in girls at risk for major depression. Arch Gen Psychiatry. 2010;67:380–7.
Leong JK, Ho TC, Colich NL, Sisk L, Knutson B, Gotlib IH. White-matter tract connecting anterior insula to nucleus accumbens predicts greater future motivation in adolescents. Dev Cogn Neurosci. 2021;47:100881.
Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–94.
Esteban O, Ciric R, Finc K, Blair RW, Markiewicz CJ, Moodie CA, et al. Analysis of task-based functional MRI data preprocessed with fMRIPrep. Nat Protoc. 2020;15:2186–2202.
Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods. 2019;16:111–6.
Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL, et al. Unbiased average age-appropriate atlases for pediatric studies. NeuroImage. 2011;54:313–27.
Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage. 2014;84:320–41.
Smyser CD, Snyder AZ, Neil JJ. Functional connectivity MRI in infants: Exploration of the functional organization of the developing brain. NeuroImage. 2011;56:1437–52.
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–S219.
Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage. 2001;14:1370–86.
Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37:90–101.
Siegel JS, Power JD, Dubis JW, Vogel AC, Church JA, Schlaggar BL, et al. Statistical improvements in functional magnetic resonance imaging analyses produced by censoring high-motion data points. Hum Brain Mapp. 2014;35:1981–96.
Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80.
R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2022.
Aiken LS, West SG, Reno RR. Multiple regression: testing and interpreting interactions. SAGE Publications, Inc. In Thousand Oaks, CA; 1991.
Johnson PO, Neyman J. Tests of certain linear hypotheses and their application to some educational problems. Stat Res Mem. 1936;1:57–93.
Reynolds WM. The reynolds adolescent depression scale-second edition (RADS-2). In: Comprehensive handbook of psychological assessment, vol. 2: personality assessment. John Wiley & Sons, Inc. in Hoboken, New Jersey; 2004. p. 224–36.
Neurauter G, Schröcksnadel K, Scholl-Bürgi S, Sperner-Unterweger B, Schubert C, Ledochowski M, et al. Chronic immune stimulation correlates with reduced phenylalanine turnover. Curr Drug Metab. 2008;9:622–7.
Chat IK, Nusslock R, Moriarity DP, Bart CP, Mac Giollabhui N, Damme K, et al. Goal-striving tendencies moderate the relationship between reward-related brain function and peripheral inflammation. Brain Behav Immun. 2021;94:60–70.
Chahal R, Miller JG, Yuan JP, Buthmann JL, Gotlib IH. An exploration of dimensions of early adversity and the development of functional brain network connectivity during adolescence: Implications for trajectories of internalizing symptoms. Dev Psychopathol. 2022;1–15.
McLaughlin KA, Weissman D, Bitrán D. Childhood adversity and neural development: a systematic review. Annu Rev Dev Psychol 2019;1:277–312.
Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–13.
Spear LP. Rewards, aversions and affect in adolescence: emerging convergences across laboratory animal and human data. Dev Cogn Neurosci. 2011;1:390–403.
Schreuders E, Braams BR, Blankenstein NE, Peper JS, Güroğlu B, Crone EA. Contributions of reward sensitivity to ventral striatum activity across adolescence and early adulthood. Child Dev. 2018;89:797–810.
Braams BR, van Duijvenvoorde ACK, Peper JS, Crone EA. Longitudinal changes in adolescent risk-taking: a comprehensive study of neural responses to rewards, pubertal development, and risk-taking behavior. J Neurosci. 2015;35:7226–38.
Hoogendam JM, Kahn RS, Hillegers MHJ, van Buuren M, Vink M. Different developmental trajectories for anticipation and receipt of reward during adolescence. Dev Cogn Neurosci. 2013;6:113–24.
Acknowledgements
We thank Anna Cichocki, Lauren Costello, Miranda Edwards, Jordan Garcia, Abigail Graber, Madelaine Graber, Melissa Hansen, Emma Jaeger, Julian Joachimsthaler, Vanessa Lopez, Amar Ojha, Holly Pham, Michelle Sanabria, Jill Segarra, Lucinda Sisk, Alexess Sosa, Megan Strickland, Giana Teresi, Johanna Walker, and Rachel Weisenburger for their assistance in data collection and management. We also thank Yael Rosenberg-Hasson and Holden Maecker for their assistance with the dried blood spot protocol. Finally, we thank all our participants and their families for participating in this research.
Funding
This research was supported by grants from the National Institute of Mental Health (R37MH101495 to IHG and K01MH117442 to TCH), the Stanford University Precision Health and Integrated Diagnostics (PHIND) Center (to IHG and TCH), the Stanford Institute for Research in the Social Sciences (IRiSS), the Stanford Psychology Norman Anderson Fund, and the National Science Foundation Graduate Research Fellowship Program (NSF GRFP) (all to JPY).
Author information
Authors and Affiliations
Contributions
JPY and IHG designed the study, JPY led the data processing and analysis, and writing; SMC contributed to data collection and processing, and TCH helped with data analysis and interpretation. All authors contributed to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yuan, J.P., Coury, S.M., Ho, T.C. et al. Early life stress moderates the relation between systemic inflammation and neural activation to reward in adolescents both cross-sectionally and longitudinally. Neuropsychopharmacol. 49, 532–540 (2024). https://doi.org/10.1038/s41386-023-01708-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-023-01708-y