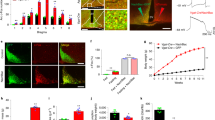
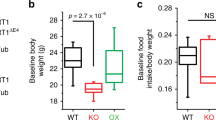
Abstract
Anorexia nervosa (AN) is a debilitating and deadly disease characterized by low body mass index due to diminished food intake, and oftentimes concurrent hyperactivity. A high percentage of AN behavioral and metabolic phenotypes can be replicated in rodents given access to a voluntary running wheel and subject to food restriction, termed activity-based anorexia (ABA). Despite the well-documented bodyweight loss observed in AN human patients and ABA rodents, much less is understood regarding the neurobiological underpinnings of these maladaptive behaviors. Hunger-promoting hypothalamic agouti-related peptide (AgRP) neurons have been well characterized in their ability to regulate appetite, yet much less is known regarding their activity and function in the mediation of food intake during ABA. Here, feeding microstructure analysis revealed ABA mice decreased food intake due to increased interpellet interval retrieval and diminished meal number. Longitudinal activity recordings of AgRP neurons in ABA animals exhibited a maladaptive inhibitory response to food, independent of basal activity changes. We then demonstrated that ABA development or progression can be mitigated by chemogenetic AgRP activation through the reprioritization of food intake (increased meal number) over hyperactivity, but only during periods of food availability. These results elucidate a potential neural target for the amelioration of behavioral maladaptations present in AN patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Diagnostic and statistical manual of mental disorders: DSM-5TM, 5th ed. 2013;5:5.
Kaye WH, Wierenga CE, Bailer UF, Simmons AN, Bischoff-Grethe A. Nothing tastes as good as skinny feels: the neurobiology of anorexia nervosa. Trends Neurosci. 2013;36:110–20.
Spring VL, Bulik CM. Implicit and explicit affect toward food and weight stimuli in anorexia nervosa. Eat Behav. 2014;15:91–94.
Kaye W. Neurobiology of anorexia and bulimia nervosa. Physiol Behav. 2008;94:121–35.
Holsen LM, Lawson EA, Blum J, Ko E, Makris N, Fazeli PK, et al. Food motivation circuitry hypoactivation related to hedonic and nonhedonic aspects of hunger and satiety in women with active anorexia nervosa and weight-restored women with anorexia nervosa. J Psychiatry Neurosci. 2012;37:322–32.
Stern SA, Bulik CM Alternative Frameworks for Advancing the Study of Eating Disorders. Trends Neurosci. 30 October 2020. https://doi.org/10.1016/j.tins.2020.10.001.
Wonderlich SA, Bulik CM, Schmidt U, Steiger H, Hoek HW. Severe and enduring anorexia nervosa: Update and observations about the current clinical reality. Int J Eat Disord. 2020;53:1303–12.
Clausen L, Jones A. A systematic review of the frequency, duration, type and effect of involuntary treatment for people with anorexia nervosa, and an analysis of patient characteristics. J Eat Disord. 2014;2:29.
Bargiacchi A, Clarke J, Paulsen A, Leger J. Refeeding in anorexia nervosa. Eur J Pediatr. 2019;178:413–22.
Mitchell JE, Peterson CB. Anorexia Nervosa. N. Engl J Med. 2020;382:1343–51.
Fichter MM, Quadflieg N. Mortality in eating disorders - results of a large prospective clinical longitudinal study. Int J Eat Disord. 2016;49:391–401.
Hardaway JA, Crowley NA, Bulik CM, Kash TL. Integrated circuits and molecular components for stress and feeding: implications for eating disorders. Genes Brain Behav. 2015;14:85–97.
Wu J, Liu J, Li S, Ma H, Wang Y. Trends in the prevalence and disability-adjusted life years of eating disorders from 1990 to 2017: results from the Global Burden of Disease Study 2017. Epidemiol Psychiatr. Science. 2020;29:e191.
Massa MG, Correa SM. Sexes on the brain: Sex as multiple biological variables in the neuronal control of feeding. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165840.
Kron L, Katz JL, Gorzynski G, Weiner H. Hyperactivity in anorexia nervosa: a fundamental clinical feature. Compr Psychiatry. 1978;19:433–40.
Achamrah N, Coëffier M, Déchelotte P. Physical activity in patients with anorexia nervosa. Nutr Rev. 2016;74:301–11.
Harris A, Hay P, Touyz S. Psychometric properties of instruments assessing exercise in patients with eating disorders: a systematic review. J Eat Disord. 2020;8:45.
Gorrell S, Flatt RE, Bulik CM, Le Grange D Psychosocial etiology of maladaptive exercise and its role in eating disorders: A systematic review. Int J Eat Disord. 4 May 2021. https://doi.org/10.1002/eat.23524.
Pirke KM, Broocks A, Wilckens T, Marquard R, Schweiger U. Starvation-induced hyperactivity in the rat: the role of endocrine and neurotransmitter changes. Neurosci Biobehav Rev. 1993;17:287–94.
Routtenberg A, Kuznesof AW. Self-starvation of rats living in activity wheels on a restricted feeding schedule. J Comp Physiol Psychol. 1967;64:414–21.
Gutierrez E. A rat in the labyrinth of anorexia nervosa: contributions of the activity-based anorexia rodent model to the understanding of anorexia nervosa. Int J Eat Disord. 2013;46:289–301.
Schalla MA, Stengel A. Activity Based Anorexia as an Animal Model for Anorexia Nervosa-A Systematic Review. Front Nutr. 2019;6:69.
Guisinger S. Adapted to flee famine: adding an evolutionary perspective on anorexia nervosa. Psychol Rev. 2003;110:745–61.
Foldi CJ, Milton LK, Oldfield BJ. A focus on reward in anorexia nervosa through the lens of the activity-based anorexia rodent model. J Neuroendocrinol. 2017;29.
Vink T, Hinney A, van Elburg AA, van Goozen SH, Sandkuijl LA, Sinke RJ, et al. Association between an agouti-related protein gene polymorphism and anorexia nervosa. Mol Psychiatry. 2001;6:325–8.
Dardennes RM, Zizzari P, Tolle V, Foulon C, Kipman A, Romo L, et al. Family trios analysis of common polymorphisms in the obestatin/ghrelin, BDNF and AGRP genes in patients with Anorexia nervosa: association with subtype, body-mass index, severity and age of onset. Psychoneuroendocrinology. 2007;32:106–13.
Moriya J, Takimoto Y, Yoshiuchi K, Shimosawa T, Akabayashi A. Plasma agouti-related protein levels in women with anorexia nervosa. Psychoneuroendocrinology. 2006;31:1057–61.
Merle JV, Haas V, Burghardt R, Döhler N, Schneider N, Lehmkuhl U, et al. Agouti-related protein in patients with acute and weight-restored anorexia nervosa. Psychol Med. 2011;41:2183–92.
de Rijke CE, Hillebrand JJG, Verhagen LAW, Roeling TAP, Adan RAH. Hypothalamic neuropeptide expression following chronic food restriction in sedentary and wheel-running rats. J Mol Endocrinol. 2005;35:381–90.
Kas MJH, van Dijk G, Scheurink AJW, Adan RAH. Agouti-related protein prevents self-starvation. Mol Psychiatry. 2003;8:235–40.
Rokot NT, Ataka K, Iwai H, Suzuki H, Tachibe H, Kairupan TS, et al. Antagonism for NPY signaling reverses cognitive behavior defects induced by activity-based anorexia in mice. Psychoneuroendocrinology. 2021;126:105133.
Hillebrand JJG, Kas MJH, Scheurink AJW, van Dijk G, Adan RAH. AgRP(83-132) and SHU9119 differently affect activity-based anorexia. Eur Neuropsychopharmacol. 2006;16:403–12.
Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121:1424–8.
Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14:351–5.
Chen Y, Lin Y-C, Zimmerman CA, Essner RA, Knight ZA. Hunger neurons drive feeding through a sustained, positive reinforcement signal. Elife. 2016;5:e18640.
Essner RA, Smith AG, Jamnik AA, Ryba AR, Trutner ZD, Carter ME. AgRP Neurons Can Increase Food Intake during Conditions of Appetite Suppression and Inhibit Anorexigenic Parabrachial Neurons. J Neurosci. 2017;37:8678–87.
Burnett CJ, Li C, Webber E, Tsaousidou E, Xue SY, Brüning JC, et al. Hunger-Driven Motivational State Competition. Neuron. 2016;92:187–201.
Burnett CJ, Funderburk SC, Navarrete J, Sabol A, Liang-Guallpa J, Desrochers TM, et al. Need-based prioritization of behavior. Elife. 2019;8:e44527.
Chen Y, Lin Y-C, Kuo T-W, Knight ZA. Sensory Detection of Food Rapidly Modulates Arcuate Feeding Circuits. Cell. 2015;160:829–41.
Beutler LR, Chen Y, Ahn JS, Lin Y-C, Essner RA, Knight ZA. Dynamics of Gut-Brain Communication Underlying Hunger. Neuron. 2017;96:461–475.e5.
Su Z, Alhadeff AL, Betley JN. Nutritive, Post-ingestive Signals Are the Primary Regulators of AgRP Neuron Activity. Cell Rep. 2017;21:2724–36.
Betley JN, Xu S, Cao ZFH, Gong R, Magnus CJ, Yu Y, et al. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature. 2015;521:180–5.
He Z, Gao Y, Alhadeff AL, Castorena CM, Huang Y, Lieu L, et al. Cellular and synaptic reorganization of arcuate NPY/AgRP and POMC neurons after exercise. Mol Metab. 2018;18:107–19.
Miletta MC, Iyilikci O, Shanabrough M, Šestan-Peša M, Cammisa A, Zeiss CJ, et al. AgRP neurons control compulsive exercise and survival in an activity-based anorexia model. Nat Metab. 2020;2:1204–11.
Beeler JA, Mourra D, Zanca RM, Kalmbach A, Gellman C, Klein BY, et al. Vulnerable and Resilient Phenotypes in a Mouse Model of Anorexia Nervosa. Biol Psychiatry. 16 July 2020. https://doi.org/10.1016/j.biopsych.2020.06.030.
Milton LK, Oldfield BJ, Foldi CJ. Evaluating anhedonia in the activity-based anorexia (ABA) rat model. Physiol Behav. 2018;194:324–32.
Milton LK, Patton T, O’Keeffe M, Oldfield BJ, Foldi CJ. In pursuit of biomarkers for predicting susceptibility to activity-based anorexia in adolescent female rats. Int J Eat Disord. 2022;55:664–77.
Hurley MM, Murlanova K, Macias LK, Sabir AI, O’Brien SC, Bhasin H, et al. Activity-based anorexia disrupts systemic oxidative state and induces cortical mitochondrial fission in adolescent female rats. Int J Eat Disord. 2021;54:639–45.
Matikainen-Ankney BA, Earnest T, Ali M, Casey E, Wang JG, Sutton AK, et al. An open-source device for measuring food intake and operant behavior in rodent home-cages. Elife. 2021;10.
Foldi CJ, Milton LK, Oldfield BJ. The Role of Mesolimbic Reward Neurocircuitry in Prevention and Rescue of the Activity-Based Anorexia (ABA) Phenotype in Rats. Neuropsychopharmacology. 2017;42:2292–2300.
Milton LK, Mirabella PN, Greaves E, Spanswick DC, van den Buuse M, Oldfield BJ, et al. Suppression of Corticostriatal Circuit Activity Improves Cognitive Flexibility and Prevents Body Weight Loss in Activity-Based Anorexia in Rats. Biol Psychiatry. 2 July 2020. https://doi.org/10.1016/j.biopsych.2020.06.022.
Verhagen LAW, Luijendijk MCM, Hillebrand JJG, Adan RAH. Food-anticipatory activity in activity-based anorexia: The involvement of dopamine. Appetite. 2007;49:337.
Daimon CM, Hentges ST. β-endorphin differentially contributes to food anticipatory activity in male and female mice undergoing activity-based anorexia. Physiol Rep. 2021;9:e14788.
Daimon CM, Hentges ST. Inhibition of POMC neurons in mice undergoing activity-based anorexia selectively blunts food anticipatory activity without affecting body weight or food intake. Am J Physiol Regul Integr Comp Physiol. 2022;322:R219–R227.
Scharner S, Prinz P, Goebel-Stengel M, Kobelt P, Hofmann T, Rose M, et al. Activity-Based Anorexia Reduces Body Weight without Inducing a Separate Food Intake Microstructure or Activity Phenotype in Female Rats-Mediation via an Activation of Distinct Brain Nuclei. Front Neurosci. 2016;10:475.
Schwartz GJ, Zeltser LM. Functional organization of neuronal and humoral signals regulating feeding behavior. Annu Rev Nutr. 2013;33:1–21.
Azevedo EP, Ivan VJ, Friedman JM, Stern SA. Higher-Order Inputs Involved in Appetite Control. Biol Psychiatry. 2022;91:869–78.
Mandelblat-Cerf Y, Ramesh RN, Burgess CR, Patella P, Yang Z, Lowell BB, et al. Arcuate hypothalamic AgRP and putative POMC neurons show opposite changes in spiking across multiple timescales. Elife. 2015;4:e07122.
Dietrich MO, Zimmer MR, Bober J, Horvath TL. Hypothalamic Agrp Neurons Drive Stereotypic Behaviors beyond Feeding. Cell. 2015;160:1222–32.
Padilla SL, Qiu J, Soden ME, Sanz E, Nestor CC, Barker FD, et al. Agouti-related peptide neural circuits mediate adaptive behaviors in the starved state. Nat Neurosci. 2016;19:734–41.
Betley JN, Cao ZFH, Ritola KD, Sternson SM. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell. 2013;155:1337–50.
Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, et al. Remote Control of Neuronal Activity in Transgenic Mice Expressing Evolved G Protein-Coupled Receptors. Neuron. 2009;63:27–39.
Zhu H, Aryal DK, Olsen RHJ, Urban DJ, Swearingen A, Forbes S, et al. Cre-dependent DREADD (Designer Receptors Exclusively Activated by Designer Drugs) mice. Genesis. 2016;54:439–46.
Klenotich SJ, Ho EV, McMurray MS, Server CH, Dulawa SC. Dopamine D2/3 receptor antagonism reduces activity-based anorexia. Transl Psychiatry. 2015;5:e613.
Welch AC, Zhang J, Lyu J, McMurray MS, Javitch JA, Kellendonk C, et al. Dopamine D2 receptor overexpression in the nucleus accumbens core induces robust weight loss during scheduled fasting selectively in female mice. Mol Psychiatry. 2021;26:3765–77.
Beutler LR, Corpuz TV, Ahn JS, Kosar S, Song W, Chen Y, et al. Obesity causes selective and long-lasting desensitization of AgRP neurons to dietary fat. Elife. 2020;9:e55909.
Mazzone CM, Liang-Guallpa J, Li C, Wolcott NS, Boone MH, Southern M, et al. High-fat food biases hypothalamic and mesolimbic expression of consummatory drives. Nat Neurosci. 2020;23:1253–66.
Lippert RN, Hess S, Klemm P, Burgeno LM, Jahans-Price T, Walton ME, et al. Maternal high-fat diet during lactation reprograms the dopaminergic circuitry in mice. J Clin Invest. 2020;130:3761–76.
Lippert RN, Brüning JC. Maternal Metabolic Programming of the Developing Central Nervous System: Unified Pathways to Metabolic and Psychiatric Disorders. Biol Psychiatry. 2022;91:898–906.
Cai X, Liu H, Feng B, Yu M, He Y, Liu H, et al. A D2 to D1 shift in dopaminergic inputs to midbrain 5-HT neurons causes anorexia in mice. Nat Neurosci. 2022;25:646–58.
Lockie SH, Stark R, Mequinion M, Ch’ng S, Kong D, Spanswick DC, et al. Glucose Availability Predicts the Feeding Response to Ghrelin in Male Mice, an Effect Dependent on AMPK in AgRP Neurons. Endocrinology. 2018;159:3605–14.
Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, et al. Agouti-related peptide–expressing neurons are mandatory for feeding. Nat Neurosci. 2005;8:1289–91.
Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP Neurons Are Essential for Feeding in Adult Mice but Can Be Ablated in Neonates. Science. 2005;310:683–5.
Goldstein N, Levine BJ, Loy KA, Duke WL, Meyerson OS, Jamnik AA, et al. Hypothalamic Neurons that Regulate Feeding Can Influence Sleep/Wake States Based on Homeostatic Need. Curr Biol. 2018;28:3736–47.
Alhadeff AL, Su Z, Hernandez E, Klima ML, Phillips SZ, Holland RA, et al. A Neural Circuit for the Suppression of Pain by a Competing Need State. Cell. 2018;173:140–152.e15.
Acknowledgements
We would like to thank Dr. Alexxai Kravitz for the development of and extensive help with FED3 devices. We thank Dr. Bryan Roth for the CNO used in chemogenetic experiments. We also thank N. Martin and B. Gloss of the NIEHS Viral Vector Core for producing the AAVs used in chemogenetic experiments. We thank all members of Dr. Krashes’ lab for technical support and guidance during this project. This research was supported by the Intramural Research Program of the National Institutes of Health, the National Institutes of Diabetes and Digestive and Kidney Diseases (DK075088 to M.J.K. and DK075087-06 to M.J.K.) and the Nancy Nossal Fellowship (NIH-NIDDK; to A.K.S.-H.).
Author information
Authors and Affiliations
Contributions
A.S.-H. and M.J.K. designed experiments. A.S.-H. and S.C.D. performed initial experiments in WT mice. A.S.-H., S.C.D., A.M.S., A.S. constructed FED3 devices used throughout experiments. A.S.-H., L.E.M. performed chemogenetic and photometry experiments. A.S.-H., E.O.K. performed behavioral experiments used in conjunction with ex vivo electrophysiology experiments. C.L. performed ex vivo electrophysiology experiments. A.S.-H., C.L. performed surgery for photometry experiments. A.K.S. performed analysis on all experiments. A.S.-H. and M.J.K. wrote the manuscript with input from S.C.D. and C.L.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Sutton Hickey, A.K., Duane, S.C., Mickelsen, L.E. et al. AgRP neurons coordinate the mitigation of activity-based anorexia. Mol Psychiatry 28, 1622–1635 (2023). https://doi.org/10.1038/s41380-022-01932-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-022-01932-w