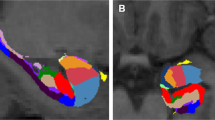
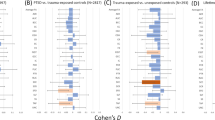
Abstract
The hippocampus and the amygdala play a central role in post-traumatic stress disorder (PTSD) pathogenesis. While alternations in volumes of both regions have been consistently observed in individuals with PTSD, it remains unknown whether these reflect pre-trauma vulnerability traits or acquired post-trauma consequences of the disorder. Here, we conducted a longitudinal panel study of adult civilian trauma survivors admitted to a general hospital emergency department (ED). One hundred eligible participants (mean age = 32.97 ± 10.97, n = 56 females) completed both clinical interviews and structural MRI scans at 1-, 6-, and 14-months after ED admission (alias T1, T2, and T3). While all participants met PTSD diagnosis at T1, only n = 29 still met PTSD diagnosis at T3 (a “non-Remission” Group), while n = 71 did not (a “Remission” Group). Bayesian multilevel modeling analysis showed robust evidence for smaller right hippocampus volume (P+ of ~0.014) and moderate evidence for larger left amygdala volume (P+ of ~0.870) at T1 in the “non-Remission” group, compared to the “Remission” group. Subregion analysis further demonstrated robust evidence for smaller volume in the subiculum and right CA1 hippocampal subregions (P+ of ~0.021–0.046) in the “non-Remission” group. No time-dependent volumetric changes (T1 to T2 to T3) were observed across all participants or between groups. Results support the “vulnerability trait” hypothesis, suggesting that lower initial volumes of specific hippocampus subregions are associated with non-remitting PTSD. The stable volume of all hippocampal and amygdala subregions does not support the idea of consequential, progressive, stress-related atrophy during the first critical year following trauma exposure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The dataset and materials used in this work is available by contacting the study PIs on a reasonable request.
Code availability
Scripts to reproduce data analysis associated with the current manuscript can be found at: https://github.com/KoremNSN/longitudinal_anatomy.
References
Liberzon I, Abelson JL. Context processing and the neurobiology of post-traumatic stress disorder. Neuron. 2016;92:14–30. https://www.sciencedirect.com/science/article/pii/S0896627316306407.
Mahan AL, Ressler KJ. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci. 2012;35:24–35.
LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nat Rev Neurosci. 2006;7:54–64. https://www.nature.com/articles/nrn1825.
van Rooij SJH, Ravi M, Ely TD, Michopoulos V, Winters SJ, Shin J, et al. Hippocampal activation during contextual fear inhibition related to resilience in the early aftermath of trauma. Behav Brain Res. 2021;408:113282.
O’Doherty DCM, Chitty KM, Saddiqui S, Bennett MR, Lagopoulos J. A systematic review and meta-analysis of magnetic resonance imaging measurement of structural volumes in posttraumatic stress disorder. Psychiatry Res Neuroimaging. 2015;232:1–33.
Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, et al. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci. 2012;13:769–87. http://www.nature.com/articles/nrn3339.
Ben-Zion Z, Artzi M, Niry D, Keynan NJ, Zeevi Y, Admon R, et al. Neuroanatomical risk factors for posttraumatic stress disorder in recent trauma survivors. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:311–9. http://biorxiv.org/content/early/2019/08/01/721134.abstract.
Kuo JR, Kaloupek DG, Woodward SH. Amygdala volume in combat-exposed veterans with and without posttraumatic stress disorder: a cross-sectional study. Arch Gen Psychiatry. 2012;69:1080–6. https://jamanetwork.com/journals/jamapsychiatry/fullarticle/1370685.
Bromis K, Calem M, Reinders AATS, Williams SCR, Kempton MJ. Meta-analysis of 89 structural MRI studies in posttraumatic stress disorder and comparison with major depressive disorder. Am J Psychiatry. 2018;175:989–98. http://ajp.psychiatryonline.org/doi/10.1176/appi.ajp.2018.17111199.
Kremen WS, Koenen KC, Afari N, Lyons MJ. Twin studies of posttraumatic stress disorder: differentiating vulnerability factors from sequelae. Neuropharmacology. 2012;62:647–53.
Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–7. https://www.nature.com/articles/nn958.
Bremner JD, Hoffman M, Afzal N, Cheema FA, Novik O, Ashraf A, et al. The environment contributes more than genetics to smaller hippocampal volume in Posttraumatic Stress Disorder (PTSD). J Psychiatr Res. 2021;137:579–88.
Admon R, Leykin D, Lubin G, Engert V, Andrews J, Pruessner J, et al. Stress-induced reduction in hippocampal volume and connectivity with the ventromedial prefrontal cortex are related to maladaptive responses to stressful military service. Hum Brain Mapp. 2013;34:2808–16. http://doi.wiley.com/10.1002/hbm.22100.
Admon R, Milad MR, Hendler T. A causal model of post-traumatic stress disorder: disentangling predisposed from acquired neural abnormalities. Trends Cogn Sci. 2013;17:337–47. https://www.sciencedirect.com/science/article/pii/S1364661313001046.
Logue MW, van Rooij SJH, Dennis EL, Davis SL, Hayes JP, Stevens JS, et al. Smaller hippocampal volume in posttraumatic stress disorder: a multisite ENIGMA-PGC study: Subcortical Volumetry Results from Posttraumatic Stress Disorder Consortia. Biol Psychiatry. 2018;83:244–53. https://pubmed.ncbi.nlm.nih.gov/29217296/.
Bonne O, Brandes D, Gilboa A, Gomori JM, Shenton ME, Pitman RK, et al. Longitudinal MRI study of hippocampal volume in trauma survivors with PTSD. Am J Psychiatry. 2001;158:1248–51. http://psychiatryonline.org/doi/abs/10.1176/appi.ajp.158.8.1248.
Xie H, Claycomb Erwin M, Elhai JD, Wall JT, Tamburrino MB, Brickman KR, et al. Relationship of hippocampal volumes and posttraumatic stress disorder symptoms over early posttrauma periods. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:968–75.
Quidé Y, Andersson F, Dufour-Rainfray D, Descriaud C, Brizard B, Gissot V, et al. Smaller hippocampal volume following sexual assault in women is associated with post-traumatic stress disorder. Acta Psychiatr Scand. 2018;138:312–24.
Mulder ER, de Jong RA, Knol DL, van Schijndel RA, Cover KS, Visser PJ, et al. Hippocampal volume change measurement: quantitative assessment of the reproducibility of expert manual outlining and the automated methods FreeSurfer and FIRST. Neuroimage. 2014;92:169–81.
Wisse LEM, Biessels GJ, Geerlings MI. A critical appraisal of the hippocampal subfield segmentation package in FreeSurfer. Front Aging Neurosci. 2014;6:261.
Amaral OB, Luft T, Cammarota M, Izquierdo I, Roesler R. Temporary inactivation of the dorsal hippocampus induces a transient impairment in retrieval of aversive memory. Behavioural Brain Res. 2007;180:113–8.
Guzowski JF, Knierim JJ, Moser EI. Ensemble dynamics of hippocampal regions CA3 and CA1. Neuron. 2004;44:581–4. https://pubmed.ncbi.nlm.nih.gov/15541306/.
Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–33. https://www.nature.com/articles/nrn2651.
Zhang L, Lu L, Bu X, Li H, Tang S, Gao Y, et al. Alterations in hippocampal subfield and amygdala subregion volumes in posttraumatic subjects with and without posttraumatic stress disorder. Hum Brain Mapp. 2021;42:2147–58. https://onlinelibrary.wiley.com/doi/full/10.1002/hbm.25356.
Chen LW, Sun D, Davis SL, Haswell CC, Dennis EL, Swanson CA, et al. Smaller hippocampal CA1 subfield volume in posttraumatic stress disorder. Depress Anxiety. 2018;35:1018–29. https://onlinelibrary.wiley.com/doi/abs/10.1002/da.22833?casa_token=iaYLPypZkKoAAAAA:eU9NURQY7wo6wogd9ZhQ6KMw7fwWPOIhlT2OTgTAHewFUuX6uMYtepmQcmIMUly5ZmoMYua8wiXgfw.
Morey RA, Clarke EK, Haswell CC, Phillips RD, Clausen AN, Mufford MS, et al. Amygdala nuclei volume and shape in military veterans with posttraumatic stress disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:281–90. https://pubmed.ncbi.nlm.nih.gov/32029420/.
Shalev AY, Freedman S. PTSD following terrorist attacks: a prospective evaluation. Am J Psychiatry. 2005;162:1188–91. http://www.ncbi.nlm.nih.gov/pubmed/15930068.
Koch SBJ, van Ast VA, Kaldewaij R, Hashemi MM, Zhang W, Klumpers F, et al. Larger dentate gyrus volume as predisposing resilience factor for the development of trauma-related symptoms. Neuropsychopharmacology. 2021;46:1283–92. https://www.nature.com/articles/s41386-020-00947-7.
Weis CN, Webb EK, Huggins AA, Kallenbach M, Miskovich TA, Fitzgerald JM, et al. Stability of hippocampal subfield volumes after trauma and relationship to development of PTSD symptoms. Neuroimage. 2021;236:118076.
Ben-Zion Z, Fine NB, Keynan NJ, Admon R, Halpern P, Liberzon I, et al. Neurobehavioral moderators of post-traumatic stress disorder (PTSD) trajectories: study protocol of a prospective MRI study of recent trauma survivors. Eur J Psychotraumatol. 2019;10:1683941. https://doi.org/10.1080/20008198.2019.1683941.
Hayes JP, Hayes S, Miller DR, Lafleche G, Logue MW, Verfaellie M. Automated measurement of hippocampal subfields in PTSD: Evidence for smaller dentate gyrus volume. J Psychiatr Res Pergamon. 2017;95:247–52.
Szeszko PR, Bierer LM, Bader HN, Chu K-W, Tang CY, Murphy KM, et al. Cingulate and hippocampal subregion abnormalities in combat-exposed veterans with PTSD. J Affect Disord. 2022;311:432–9.
Wang Z, Neylan TC, Mueller SG, Lenoci M, Truran D, Marmar CR, et al. Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Arch Gen Psychiatry. 2010;67:296–303. https://jamanetwork.com/journals/jamapsychiatry/fullarticle/210641.
Veer IM, Oei NYL, van Buchem MA, Spinhoven P, Elzinga BM, Rombouts SARB. Evidence for smaller right amygdala volumes in posttraumatic stress disorder following childhood trauma. Psychiatry Res Neuroimaging. 2015;233:436–42.
Akiki TJ, Averill CL, Wrocklage KM, Schweinsburg B, Scott JC, Martini B, et al. The association of PTSD symptom severity with localized hippocampus and amygdala abnormalities. Chronic Stress. 2017;1. https://pubmed.ncbi.nlm.nih.gov/28825050/.
Ben-Zion Z, Shany O, Admon R, Keynan NJ, Avisdris N, Balter SR, et al. Neural responsivity to reward versus punishment shortly after trauma predicts long-term development of posttraumatic stress symptoms. Biol Psychiatry Cogn Neurosci Neuroimaging. 2022;7:150–61. https://pubmed.ncbi.nlm.nih.gov/34534702/.
Schultebraucks K, Ben-Zion Z, Admon R, Keynan JN, Liberzon I, Hendler T, et al. Assessment of early neurocognitive functioning increases the accuracy of predicting chronic PTSD risk. Mol Psychiatry. 2022. https://www.nature.com/articles/s41380-022-01445-6.
Ben-Zion Z, Zeevi Y, Keynan NJ, Admon R, Kozlovski T, Sharon H, et al. Multi-domain potential biomarkers for post-traumatic stress disorder (PTSD) severity in recent trauma survivors. Transl Psychiatry. 2020;10:1–11. https://doi.org/10.1038/s41398-020-00898-z.
Hoge CW, Yehuda R, Castro CA, McFarlane AC, Vermetten E, Jetly R, et al. Unintended consequences of changing the definition of posttraumatic stress disorder INDSM-5 critique and call for action. JAMA Psychiatry. 2016;73:750–2. https://jamanetwork.com/journals/jamapsychiatry/article-abstract/2524846.
Hoge CW, Riviere LA, Wilk JE, Herrell RK, Weathers FW. The prevalence of post-traumatic stress disorder (PTSD) in US combat soldiers: a head-to-head comparison of DSM-5 versus DSM-IV-TR symptom criteria with the PTSD checklist. Lancet Psychiatry. 2014;1:269–77. https://www.sciencedirect.com/science/article/pii/S2215036614702354.
Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. http://www.springerlink.com/index/10.1007/BF02105408.
Weathers FW, Bovin MJ, Lee DJ, Sloan DM, Schnurr PP, Kaloupek DG, et al. The Clinician-Administered PTSD Scale for DSM–5 (CAPS-5): development and initial psychometric evaluation in military veterans. Psychol Assess. 2018;30:383–95.
Jackson CE, Currao A, Fonda JR, Kenna A, Milberg WP, McGlinchey RE, et al. Research utility of a CAPS-IV and CAPS-5 hybrid interview: posttraumatic stress symptom and diagnostic concordance in recent-era U.S. veterans. J Trauma Stress. 2021. https://pubmed.ncbi.nlm.nih.gov/34973042/.
Stein DJ, McLaughlin KA, Koenen KC, Atwoli L, Friedman MJ, Hill ED, et al. DSM-5 and ICD-11 definitions of posttraumatic stress disorder: Investigating “narrow” and “broad” approaches. Depress Anxiety. 2014;31:494–505. http://www.ncbi.nlm.nih.gov/pubmed/24894802.
Weathers FW, Ruscio AM, Keane TM. Psychometric properties of nine scoring rules for the clinician- administered posttraumatic stress disorder scale. Psychol Assess. 1999;11:124–33.
Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61:1402–18.
Fischl B. FreeSurfer. Neuroimage. 2012;62:774–81.
Iglesias JE, van Leemput K, Augustinack J, Insausti R, Fischl B, Reuter M. Bayesian longitudinal segmentation of hippocampal substructures in brain MRI using subject-specific atlases. Neuroimage. 2016;141:542–55.
Iglesias JE, Augustinack JC, Nguyen K, Player CM, Player A, Wright M, et al. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage. 2015;115:117–37.
Saygin ZM, Kliemann D, Iglesias JE, van der Kouwe AJW, Boyd E, Reuter M, et al. High-resolution magnetic resonance imaging reveals nuclei of the human amygdala: manual segmentation to automatic atlas. Neuroimage. 2017;155:370–82.
Woon FL, Sood S, Hedges DW. Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1181–8. https://pubmed.ncbi.nlm.nih.gov/20600466/.
Nelson MD, Tumpap AM. Posttraumatic stress disorder symptom severity is associated with left hippocampal volume reduction: a meta-analytic study. CNS Spectr. 2017;22:363–72. https://pubmed.ncbi.nlm.nih.gov/27989265/.
Tae WS, Kim SS, Lee KU, Nam EC, Kim KW. Validation of hippocampal volumes measured using a manual method and two automated methods (FreeSurfer and IBASPM) in chronic major depressive disorder. Neuroradiology. 2008;50:569–81. http://link.springer.com/10.1007/s00234-008-0383-9.
Sánchez-Benavides G, Gómez-Ansón B, Sainz A, Vives Y, Delfino M, Peña-Casanova J. Manual validation of FreeSurfer’s automated hippocampal segmentation in normal aging, mild cognitive impairment, and Alzheimer Disease subjects. Psychiatry Res Neuroimaging. 2010;181:219–25. https://www.sciencedirect.com/science/article/pii/S0925492709002443.
Wenger E, Mårtensson J, Noack H, Bodammer NC, Kühn S, Schaefer S, et al. Comparing manual and automatic segmentation of hippocampal volumes: reliability and validity issues in younger and older brains. Hum Brain Mapp. 2014;35:4236–48. https://onlinelibrary.wiley.com/doi/abs/10.1002/hbm.22473.
Schoemaker D, Buss C, Head K, Sandman CA, Davis EP, Chakravarty MM, et al. Hippocampus and amygdala volumes from magnetic resonance images in children: assessing accuracy of FreeSurfer and FSL against manual segmentation. Neuroimage. 2016;129:1–14.
Weathers FW, Huska JA, Keane TM. The PTSD Checklist-Civilian Version (PCL-C). Boston, MA: National Center for PTSD; 1991, 1994.
Limbachia C, Morrow K, Khibovska A, Meyer C, Padmala S, Pessoa L. Controllability over stressor decreases responses in key threat-related brain areas. Commun Biol. 2021;4:1–11.
Chen G, Xiao Y, Taylor PA, Rajendra JK, Riggins T, Geng F, et al. Handling multiplicity in neuroimaging through Bayesian lenses with multilevel modeling. Neuroinformatics. 2019;17:515–45.
McElreath R. Statistical rethinking: a Bayesian course with examples in R and Stan. New York: Chapman and Hall/CRC; 2018. p. 1–469.
Gelman A, Hill J. Data analysis using regression and multilevel/hierarchical models. Cambridge: Cambridge University Press; 2006.
Gelman A, Hill J, Yajima M. Why we (usually) don’t have to worry about multiple comparisons. J Res Educ Eff. 2012;5:189–211. https://www.tandfonline.com/doi/abs/10.1080/19345747.2011.618213?casa_token=Me9Li5bNQ0cAAAAA:1MjfztU3T9JEWjFQRtlbCEG75yeLOsdH0SidrcOuhinuipX2HjM7vnuPhnJTR7VtaQvU9S_jt62_9w.
Shalev AY, Ankri Y, Israeli-Shalev Y, Peleg T, Adessky R, Freedman S. Prevention of posttraumatic stress disorder by early treatment: results from the Jerusalem trauma outreach and prevention study. Arch Gen Psychiatry. 2012;69:166–76. http://www.ncbi.nlm.nih.gov/pubmed/21969418.
Szeszko PR, Lipsky R, Mentschel C, Robinson D, Gunduz-Bruce H, Sevy S, et al. Brain-derived neurotrophic factor val66met polymorphism and volume of the hippocampal formation. Mol Psychiatry. 2005;10:631–6.
Samplin E, Ikuta T, Malhotra AK, Szeszko PR, DeRosse P. Sex differences in resilience to childhood maltreatment: effects of trauma history on hippocampal volume, general cognition and subclinical psychosis in healthy adults. J Psychiatr Res. 2013;47:1174–9. https://pubmed.ncbi.nlm.nih.gov/23726669/.
Humphreys KL, King LS, Sacchet MD, Camacho MC, Colich NL, Ordaz SJ, et al. Evidence for a sensitive period in the effects of early life stress on hippocampal volume. Dev Sci. 2019;22:e12775. https://onlinelibrary.wiley.com/doi/full/10.1111/desc.12775.
de Melo MB, Favaro VM, Oliveira MGM. The dorsal subiculum is required for contextual fear conditioning consolidation in rats. Behav Brain Res. 2020;390:112661.
Maren S. Neurotoxic or electrolytic lesions of the ventral subiculum produce deficits in the acquisition and expression of Pavlovian fear conditioning in rats. Behav Neurosci. 1999;113:283–90. https://pubmed.ncbi.nlm.nih.gov/10357453/.
O’Mara S. Controlling hippocampal output: the central role of subiculum in hippocampal information processing. Behav Brain Res. 2006;174:304–12.
Herman JP, Mueller NK. Role of the ventral subiculum in stress integration. Behav Brain Res. 2006;174:215–24.
Radley JJ, Sawchenko PE. A common substrate for prefrontal and hippocampal inhibition of the neuroendocrine stress response. J Neurosci. 2011;31:9683–95. https://pubmed.ncbi.nlm.nih.gov/21715634/.
Teicher MH, Anderson CM, Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus,and subiculum. Proc Natl Acad Sci USA. 2012;109. www.pnas.org/cgi/doi/10.1073/pnas.1115396109.
Bartsch T, Döhring J, Rohr A, Jansen O, Deuschl G. CA1 neurons in the human hippocampus are critical for autobiographical memory, mental time travel, and autonoetic consciousness. Proc Natl Acad Sci USA. 2011;108:17562–7.
Postel C, Mary A, Dayan J, Fraisse F, Vallée T, Guillery-Girard B, et al. Variations in response to trauma and hippocampal subfield changes. Neurobiol Stress. 2021;15:100346.
Roy DS, Kitamura T, Okuyama T, Ogawa SK, Sun C, Obata Y, et al. Distinct neural circuits for the formation and retrieval of episodic memories. Cell. 2017;170:1000–12.e19. http://www.cell.com/article/S0092867417308206/fulltext.
van Rooij SJH, Smith RD, Stenson AF, Ely TD, Yang X, Tottenham N, et al. Increased activation of the fear neurocircuitry in children exposed to violence. Depress Anxiety. 2020;37:303.
Butler O, Herr K, Willmund G, Gallinat J, Kuhn S, Zimmermann P. Trauma, treatment and Tetris: video gaming increases hippocampal volume in male patients with combat-related posttraumatic stress disorder. J Psychiatry Neurosci. 2020;45:279–87. https://www.jpn.ca/content/45/4/279.
Sekiguchi A, Sugiura M, Taki Y, Kotozaki Y, Nouchi R, Takeuchi H, et al. Brain structural changes as vulnerability factors and acquired signs of post-earthquake stress. Mol Psychiatry. 2013;18:618–23. https://pubmed.ncbi.nlm.nih.gov/22614289/.
Sekiguchi A, Kotozaki Y, Sugiura M, Nouchi R, Takeuchi H, Hanawa S, et al. Resilience after 3/11: structural brain changes 1 year after the Japanese earthquake. Mol Psychiatry. 2014;20:553–4. https://www.nature.com/articles/mp201428.
Liu H, Petukhova MV, Sampson NA, Aguilar-Gaxiola S, Alonso J, Andrade LH, et al. Association of DSM-IV posttraumatic stress disorder with traumatic experience type and history in the World Health Organization World Mental Health Surveys. JAMA Psychiatry. 2017;74:270–81. https://jamanetwork.com/journals/jamapsychiatry/fullarticle/2595039.
Hinojosa CA. Does hippocampal volume in patients with posttraumatic stress disorder vary by trauma type? Harv Rev Psychiatry. 2022;30:118. https://journals.lww.com/hrpjournal/Fulltext/2022/03000/Does_Hippocampal_Volume_in_Patients_with.3.aspx.
Galatzer-Levy IR, Bryant RA. 636,120 ways to have posttraumatic stress disorder. Perspect Psychol Sci. 2013;8:651–62.
Zoellner LA, Bedard-Gilligan MA, Jun JJ, Marks LH, Garcia NM. The evolving construct of posttraumatic stress disorder (PTSD): DSM-5 criteria changes and legal implications. Psychol Inj Law. 2013;6:277–89. http://link.springer.com/10.1007/s12207-013-9175-6.
Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the national comorbidity survey. Arch Gen Psychiatry. 1995;52:1048–60. http://archpsyc.jamanetwork.com/article.aspx?doi=10.1001/archpsyc.1995.03950240066012.
Wisse LEM, Chételat G, Daugherty AM, de Flores R, la Joie R, Mueller SG, et al. Hippocampal subfield volumetry from structural isotropic 1 mm3 MRI scans: a note of caution. Hum Brain Mapp. 2021;42:539–50. https://onlinelibrary.wiley.com/doi/full/10.1002/hbm.25234.
Acknowledgements
The authors would like to thank our wonderful research team at Tel-Aviv Sourasky Medical Center—including Naomi Fine, Nili Green, Mor Halevi, Sheli Luvton, Yael Shavit, Olga Nevenchannaya, Iris Rashap, Efrat Routledge, and Ophir Leshets—for their significant contributions to participants, screening, enrollment, assessments, and follow-up. We extend our gratitude to all the participants of this study, who completed all the assessments at three different time points after experiencing a traumatic event, thus contributing to scientific research on post-traumatic psychopathology.
Funding
This work was supported by award number R01-MH-103287 from the National Institute of Mental Health (NIMH) given to AYS (PI), IL, and TH (co-Investigators, subcontractors), and had undergone critical review by the NIMH Adult Psychopathology and Disorders of Aging study section.
Author information
Authors and Affiliations
Contributions
TH, IL, and AYS designed, obtained funding, supervised, and oversaw the implementation of the study. ZB-Z, AYS, and TH had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. ZB-Z, JNK, and RA carried out the procedural aspects of the study, the research assistants’ training, guidance and monitoring, management of participants, and QA of data. ZB-Z, NK, OD, and TRS analyzed the results and drafted the initial manuscript together with IH-R, AYS, and TH. Critical revisions of the manuscript were conducted by NK, OD, TRS, JNK, RA, IH-R, IL, AYS, and TH, until finalizing it. All authors have read and approved the final manuscript and its submission to “Molecular Psychiatry”.
Corresponding author
Ethics declarations
Competing interests
TH is the chief medical officer of “GrayMatters Health Co” (Haifa, Israel). All other authors report no potential conflicts of interest to declare.
Ethical approval
The research study met all ethical regulations as required by the ethics committee at Tel-Aviv Sourasky Medical Center (Reference number 0207/14). All subjects gave written informed consent in accordance with the Declaration of Helsinki and received financial remuneration at the end of each time-point (1-, 6-, and 14-months post-trauma). The study’s ClinicalTrials.gov registration ID is NCT03756545 (clinicaltrials.gov/ct2/show/NCT03756545).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ben-Zion, Z., Korem, N., Spiller, T.R. et al. Longitudinal volumetric evaluation of hippocampus and amygdala subregions in recent trauma survivors. Mol Psychiatry 28, 657–667 (2023). https://doi.org/10.1038/s41380-022-01842-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-022-01842-x