Abstract
Numerous risk factors for mental disorders have been identified. However, we do not know how many disorders we could prevent and to what extent by modifying these risk factors. This study quantifies the Population Attributable Fraction (PAF) of potentially modifiable risk factors for mental disorders. We conducted a PRISMA 2020-compliant (Protocol: https://osf.io/hk2ag) meta-umbrella systematic review (Web of Science/PubMed/Cochrane Central Register of Reviews/Ovid/PsycINFO, until 05/12/2021) of umbrella reviews reporting associations between potentially modifiable risk factors and ICD/DSM mental disorders, restricted to highly convincing (class I) and convincing (class II) evidence from prospective cohorts. The primary outcome was the global meta-analytical PAF, complemented by sensitivity analyses across different settings, the meta-analytical Generalised Impact Fraction (GIF), and study quality assessment (AMSTAR). Seven umbrella reviews (including 295 meta-analyses and 547 associations) identified 28 class I–II risk associations (23 risk factors; AMSTAR: 45.0% high-, 35.0% medium-, 20.0% low quality). The largest global PAFs not confounded by indication were 37.84% (95% CI = 26.77–48.40%) for childhood adversities and schizophrenia spectrum disorders, 24.76% (95% CI = 13.98–36.49%) for tobacco smoking and opioid use disorders, 17.88% (95% CI = not available) for job strain and depression, 14.60% (95% CI = 9.46–20.52%) for insufficient physical activity and Alzheimer’s disease, 13.40% (95% CI = 7.75–20.15%) for childhood sexual abuse and depressive disorders, 12.37% (95% CI = 5.37–25.34%) for clinical high-risk state for psychosis and any non-organic psychotic disorders, 10.00% (95% CI = 5.62–15.95%) for three metabolic factors and depression, 9.73% (95% CI = 4.50–17.30%) for cannabis use and schizophrenia spectrum disorders, and 9.30% (95% CI = 7.36–11.38%) for maternal pre-pregnancy obesity and ADHD. The GIFs confirmed the preventive capacity for these factors. Addressing several potentially modifiable risk factors, particularly childhood adversities, can reduce the global population-level incidence of mental disorders.
Similar content being viewed by others

Introduction
A large-scale meta-analysis found that the global onset of the first mental disorder occurs before age 14 in one-third (34.6%), before age 18 in half (48.4%), and before age 25 in almost two-thirds (62.5%) of cases, with a peak onset age of 14.5 years and a median age at onset of 18 years across all mental disorders [1]. Due to the suboptimal efficacy of interventions after the onset of mental disorders [2], primary prevention is particularly promising in young people [3]. It encompasses: (i) targeted strategies in individuals at clinical high risk (indicated interventions) [4,5,6] or those asymptomatic who have significant risk factors (selective interventions) [7,8,9], or (ii) public health strategies in the general population (universal interventions) [7].
Primary prevention requires a robust aetiopathological knowledge of the natural history of a disorder [10], but mental disorders are intrinsically complex conditions. Although a genetic predisposition is evident, it explains only a small proportion of the phenotypic variance [11,12,13]; environmental factors underlie much of the phenotypic variation [14]. Individual studies exploring non-purely genetic risk factors for mental disorders have grown over the past decades, to the point that numerous umbrella reviews (i.e., systematic reviews of meta-analyses [15,16,17,18]) have summarised the consistency and magnitude of these risk factors [19,20,21,22]. As umbrella reviews can robustly rank the credibility of the evidence [23], controlling at the same time for several biases [19,20,21,22], they are considered at the top of the hierarchy to evaluate epidemiological evidence [18, 24]. Despite these potentials, the associations reported by umbrella reviews are not directly informative for preventive interventions. Unmasking the power of preventative approaches [25] requires assessing the proportional reduction in population-level disease (Population Attributable Fraction, PAF) [26,27,28,29] that would occur if a given risk factor is eliminated in an ideal exposure scenario [29] (https://www.who.int/healthinfo/global_burden_disease/metrics_paf/en/). The PAF, which is influenced by the prevalence of the exposure (risk factor), estimates the epidemiologic contribution of a risk factor to a certain disease [30], informing the prioritisation of preventive targets across diverse prevalence settings (e.g. in low–middle-income countries or in specific sociodemographic groups) [29]. To our best knowledge, no study has estimated the meta-analytic PAF of the most robust risk factors for mental disorders.
We fill this gap by quantifying the consistency and magnitude of the PAF for the most robust non-purely genetic and potentially modifiable risk factors across all mental disorders. We combined published umbrella reviews ranking robust risk factors for mental disorders with global population-level prevalence data and bespoke meta-analytical methods.
Methods
Search strategy and selection criteria
We conducted a PRISMA 2020-compliant [31, 32] (eMethods 1) meta-umbrella systematic review; an umbrella review of umbrella reviews [33] (protocol: https://osf.io/hk2ag). Two researchers (ED, MS) independently searched Web of Science (Clarivate Analytics) databases (including the Web of Science Core Collection/BIOSIS Citation Index/MEDLINE/KCI-Korean Journal Database/SciELO Citation Index/Russian Science Citation Index), PubMed, the Cochrane Central Register of Reviews, and Ovid/PsycINFO databases, from inception to 05/12/2021, using: “umbrella review” and (“risk” OR “protect*”, see eBox1). Records identified were screened based on title and abstract; full texts of the relevant records were assessed for inclusion. The references of records included were additionally screened.
Studies included were: (a) umbrella reviews [16, 17]; (b) reporting quantitative data from prospective cohort studies on the association between non-purely genetic risk factors and (ICD/DSM-any version) mental disorders, based on established criteria for classifying the credibility of the evidence [19,20,21,22] (see below).
Studies excluded were: (a) systematic reviews or meta-analyses other than quantitative umbrella reviews, individual studies, clinical cases, conference proceedings, and study protocols; (b) umbrella reviews addressing outcomes other than the onset of an established mental disorder (e.g., those related to clinical outcomes such as relapse, remission or treatment response [34, 35]); (c) umbrella reviews employing other classification approaches, such as GRADE [36], because these umbrellas do not present quantitative results from prospective cohort studies only; (d) umbrella reviews addressing pure genetic factors or biomarkers because genetic/biomarker association is tested with other analytical approaches.
Corresponding authors were contacted to clarify data overlaps. When two papers presented overlapping datasets on the same risk factor for the same disorder, only the paper with the largest dataset was retained.
Measures and data extraction
Two of us (ED, MS) independently extracted a predetermined set of variables characterising each umbrella review, including first author and publication year, number of meta-analyses included, median number of individual studies and cases (with interquartile range) per association in each meta-analysis included, the overall number of risk factors investigated, and the range of years for which the evidence was reviewed.
Additional variables were extracted to characterise the association between risk factors and mental disorders. Each risk factor was pragmatically defined as originally operationalized by each individual study, without redefining it unless strictly necessary to improve reporting clarity (eTable 1). Since each risk factor (e.g., smoking) can be associated with multiple outcomes (e.g., lung and pancreatic cancer), the total number of risk associations tested in umbrella reviews typically exceeds the number of risk factors [37].
We also recorded the specific mental disorder and matched it with the corresponding ICD-10 diagnostic block (eMethods 2). Furthermore, we recorded the number of individual studies and cases analysed per association and the association’s strength as risk ratios (RRs) ± 95% confidence intervals (CIs). Finally, we extracted the class of evidence (class I or II) [13, 18,19,20, 38] as reported for each association (see below), but only focused on risk factors (protective factors were reversed) that: (i) could be potentially modifiable as clinically evaluated, (ii) were not affected by survival bias, and (iii) were derived from prospective cohort analyses. The latter criterion was applied to specifically deal with the problem of reverse causation that may affect, for example, case–control studies [21].
Strategy for data synthesis
We presented the associations stratified across the corresponding ICD-10 diagnostic blocks. The classification of the credibility of the evidence was defined according to established criteria [13, 18,19,20, 38]: prospective class I, convincing (number of cases >1000, P < 10−6, I2 < 50%, 95% prediction interval excluding the null, no small-study effects, and no excess significance bias); prospective class II, highly suggestive (number of cases >1000, P < 10−6, largest study with a statistically significant effect, and class I criteria not met). We indicated whether there could be confounding by indication, e.g., associations between a medical treatment and a mental disorder could be confounded by an underlying medical condition, which would have increased the indication for medical treatment and the risk of the mental disorder [39]. We recorded the quality of the included meta-analyses using the AMSTAR (A Measurement Tool to Assess Systematic Reviews) tool, as reported in original umbrella reviews [40].
The global PAF analysis (primary outcome) was then conducted [29]. To retrieve robust prevalence data (±95% CIs), we adopted a systematic multistep approach. We preferably used estimates from the Global Burden of Disease Study (GBD, 2019) (http://ghdx.healthdata.org), followed by GBD 2015, a catalogue of global health, causes, demographic data, and vital statistics for both global and county profiles previously established in epidemiological research [41,42,43,44,45]. When GBD prevalences were not available, we favoured global reports of population-level prevalences by international agencies (e.g., World Health Organization, Centers for Disease Control and Prevention, European Centre for Disease Prevention and Control, etc.) [46] (https://data.cdc.gov; https://www.ecdc.europa.eu/en), followed by meta-analyses/systematic reviews, and then individual population-based studies (eTables 2 and 3). Additional computations were also performed to generate prevalence data as detailed in eMethods 3 and eFigures 1–4. All primary analyses followed the pre-specified protocol. Sensitivity analyses additionally tested the impact of variable prevalence in different settings (eMethods 4).
We appraised the quality of individual studies reporting prevalence using a modified version of a critical appraisal tool for systematic reviews addressing prevalence items [47].
The calculation of the PAF was based on Levin’s formula [48], which requires the RR estimate and the prevalence (P) of the risk factor [49].
Even if odds ratios (OR) are very similar to RRs when the incidence of an outcome is low, we preferred converting all ORs to RRs using a standard formula [50]. In all, 95% CIs for the PAFs were derived using a method similar to Daly’s [51]. Specifically, for each risk factor, we created 50,000 random RRs according to the RR 95%CI and 50,000 random prevalences according to the prevalence 95% CI. We then combined the random RRs and prevalences to derive 50,000 PAF estimations, from which we derived the PAF 95% CI.
While the PAF assumes a perfect intervention that eradicates the exposure (i.e. 100% reduction of the prevalence of the risk factors) [30], complete removal of exposure is often unrealistic. Therefore, we performed additional (secondary) analyses by computing the GIF (Generalised Impact Fraction, also called the generalised attributable fraction) for factors with the largest PAFs (as the GIF is ≤PAF, for smaller PAFs, the GIF analysis would be futile) and not confounded by indication. The GIF estimates the proportional reduction in disease incidence given a graded reduction in the prevalence of a risk factor [30, 52].
All analyses were conducted using Stata 17 (StataCorp. 2017 Stata Statistical Software: Release 17. College Station, TX) and R (version 4.0.3).
Results
Database
Overall, 2278 records were retrieved, 1382 suitable papers were screened after duplicates were removed, and seven umbrella reviews were finally included after examining 68 for depth eligibility [17, 37, 53,54,55,56,57]. (see Fig. 1 and eTable 4). Included umbrella reviews were published 2017–2021, with individual studies published 1995–2020. The seven eligible umbrella reviews (eTable 5) included 295 meta-analyses (median = 43, interquartile range = 35–55) and 547 associations between putative risk factors and mental disorders that were analysed.
Characteristics of the included umbrella reviews
Among the 547 associations, 30 were of class I, 40 were of class II, 70 were of class III, 227 of class IV, and 180 were non-significant in the main analysis. However, only 28 risk associations of class I–II (16 class I and 12 class II), relating to 23 risk factors (eTable 2 for definitions) from 20 meta-analyses survived in prospective analysis (after excluding non-modifiable risk factors, such as widowhood, and factors affected by survival biases, such as the history of cancer), and were included in the current study. Table 1 summarises the associations of the 23 risk factors and mental disorders that have been included in the current study, stratified by ICD-10 diagnostic blocks.
Quality assessment
Based on the AMSTAR evaluation, nine meta-analyses (45.0%) reported on 12 associations were of high quality, seven meta-analyses (35.0%) reported on nine associations of medium-quality, and four meta-analyses (20.0%) reported on seven associations of low quality (Table 1). The main methodological differences between high/medium and low-quality reviews are described in eResults 1.
Evidence for the association between risk factors and mental disorders in prospective studies
Organic, including symptomatic, mental disorders
The seven class I associations and five risk factors included (Table 1): type 2 diabetes mellitus (T2DM) (vascular dementia, RR = 2.28, 95% CI 1.94–2.66, and Alzheimer’s disease, RR = 1.54, 95% CI 1.39–1.72); depression (any dementia, RR = 1.86, 95% CI 1.61–2.14); depression in elderhood (any dementia, RR = 1.83, 95% CI 1.65–2.03, and Alzheimer’s disease, RR = 1.64, 95% CI 1.40–1.92); low frequency of social contacts (any dementia, RR = 1.57, 95% CI 1.32–1.85); and benzodiazepine use (any dementia, RR = 1.49, 95% CI 1.30–1.72; likely confounding by indication including difficulties with sleep and chronic anxiety with or without depression).
Three class II associations and three risk factors included (Table 1): depression (Alzheimer’s disease, RR = 1.72, 95% CI 1.39–2.13), insufficient physical activity (reversed protective factor, Alzheimer’s disease, RR = 1.62, 95% CI 1.38–1.91; eFigure 1) and T2DM (any dementia, RR = 1.60, 95% CI 1.43–1.79).
Mental and behavioural disorders due to psychoactive substance use
No association was supported by class I evidence (Table 1). Only one class II association involved tobacco smoking as a risk factor for opioid use disorder (RR = 2.61, 95% CI = 1.79–3.79).
Schizophrenia, schizotypal, and delusional disorders
Only one class I association included (Table 1): clinical high-risk state for psychosis (CHR-P) as a risk factor for any non-organic psychotic disorder (RR = 9.30, 95% CI 4.91–17.66).
Two class II associations included (Table 1): cannabis use (RR = 3.84, 95% CI 2.34–6.29) and childhood adversities (RR = 2.57, 95% CI 1.94–3.40) for schizophrenia spectrum disorders.
Mood (affective) disorders
Five class I associations of five risk factors for depressive disorders included (Table 1): sexual dysfunction (RR = 2.49, 95% CI not available), four or five metabolic risk factors (RR = 1.98, 95% CI 1.56–2.53), childhood physical abuse (RR = 1.89, 95% CI 1.70–2.09), job strain (RR = 1.73, 95% CI 1.44–2.06), and obesity (RR = 1.33, 95% CI 1.20–1.47).
Three class II associations included (Table 1): childhood sexual abuse (RR = 2.31, 95% CI 1.72–3.10) and three metabolic risk factors (RR = 1.93, 95% CI 1.57–2.37) as risk factors for depressive disorders, and sleep disturbances as a risk factor for depressive disorders in elderhood (RR = 1.92, 95% CI 1.59–2.33).
Neurotic, stress-related and somatoform disorders, behavioural syndromes associated with physiological disturbances and physical factors, disorders of adult personality and behaviour, mental retardation
No class I–II associations/risk factors were identified.
Disorders of psychological development
Two class II associations (Table 1) involved two risk factors for autism spectrum disorder: maternal selective serotonin-reuptake inhibitor (SSRI) use during pregnancy (RR = 1.65, 95% CI 1.37–2.00, confounding by indication, such as underlying maternal mental disorders) and maternal overweight pre/during pregnancy (RR = 1.30, 95% CI 1.21–1.40).
Behavioural and emotional disorders with onset usually occurring in childhood and adolescence
Three class I associations (Table 1) included three risk factors for ADHD: maternal obesity pre-pregnancy (RR = 1.63, 95% CI 1.49–1.78), maternal overweight pre/during pregnancy (RR = 1.28, 95% CI 1.20–1.36), and maternal paracetamol use during pregnancy (RR = 1.25, 95% CI 1.17–1.34, likely confounding by indication). One class II association (Table 1) involved maternal smoking during pregnancy as a risk factor for ADHD (RR = 1.60, 95% CI 1.41–1.75).
Global meta-analytic PAF of risk factors for mental disorders
The global meta-analytic PAFs for each mental disorder (in decreasing order of magnitude) with the associated global prevalence (for full prevalence data, see eResults 2 and eTable 2) are presented in Table 2. The PAF of vascular dementia associated with T2DM was 6.73% (95% CI = 5.01–8.72); the PAF of any dementia associated with benzodiazepine use was 5.84% (95% CI = 3.61–8.30), with depression in elderhood 4.30% (95% CI = 3.21–5.60), with T2DM 3.28% (95% CI = 2.35–4.34), and with depression 3.00% (95% CI = 2.13–4.03). The PAF of Alzheimer’s disease associated with insufficient physical activity was 14.60% (95% CI = 9.46–20.52), with depression in elderhood 3.35% (95% CI = 2.06–4.92), with T2DM 2.98% (95% CI = 2.12–3.95), and with depression 2.53% (95% CI = 1.38–3.97). GIF analysis showed that insufficient physical activity should be reduced by 68% to prevent 10% of Alzheimer’s disease (eFigure 5).
The PAF of opioid use disorder associated with tobacco smoking was 24.76% (95% CI = 13.98–36.49). GIF analysis showed that tobacco smoking should be reduced by 40% to prevent 10% of opioid use disorder cases (eFigure 6).
The PAF of schizophrenia spectrum disorders associated with childhood adversities and cannabis use were 37.84% (95% CI = 26.77–48.40) and 9.73% (95% CI = 4.50–17.30), respectively, while the PAF of any non-organic psychotic disorders associated with the CHR-P was 12.37% (95% CI = 5.37–25.34). GIF analyses showed that childhood adversities should be reduced by 26%, or CHR-P by 81% or cannabis use by 100% to prevent 10% of schizophrenia spectrum disorders (Fig. 2).
The PAF of depressive disorders associations was 17.88% (95% CI = not calculatable) with job strain, 13.40% (95% CI = 7.75–20.15) with childhood sexual abuse, 10.0% (95% CI = 5.62–15.95) with three metabolic risk factors, 6.60% (95% CI = 5.30–8.01) with childhood physical abuse, 4.69% (95% CI = 2.26–9.24) with four or five metabolic risk factors, and 2.64% (95% CI = 1.63–3.74) with obesity. GIF analyses showed that job strain should be reduced by 56%, or childhood sexual abuse by 75% or having three metabolic risk factors by 100% to prevent 10% of depressive disorders (eFigures 7 and 8).
The PAF of autism spectrum disorder associations was 6.47% (95% CI = 4.59–8.41), with maternal overweight pre/during pregnancy and 1.93% (95% CI = 1.02–3.08) with maternal SSRI use during pregnancy.
The PAF of ADHD associations was 10.15% (95% CI = 6.72–13.74) with maternal paracetamol use during pregnancy, 9.30% (95% CI = 7.36–11.38) with maternal pre-pregnancy obesity, 6.02% (95% CI = 4.40–7.68) with maternal overweight pre/during pregnancy, and 0.98% (95% CI = 0.36–2.66) with maternal smoking during pregnancy. The GIF for maternal pre-pregnancy obesity is illustrated in eFigure 9.
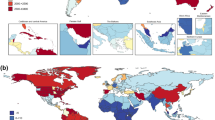
Additional sensitivity analyses are reported in the eResults 3, eFigures 5–11, and eTable 6; an illustrative world map of the country-level prevalence of cannabis use is presented in Fig. 3.
Discussion
We estimated for the first time the global meta-analytic PAFs of 23 robust, potentially modifiable risk factors for mental disorders of class I–II evidence, as published in seven umbrella reviews summarising 295 meta-analyses and 547 associations. These results provide essential epidemiological knowledge that can deconstruct the relative contribution of risk factors to the incidence of mental disorders and inform preventive approaches. By applying the largest literature synthesis and adopting stringent evidence-based classification criteria to rank associations, we identified nine potentially modifiable risk factors with a large PAF (and not confounded by indication) that can be targeted to reduce the global incidence of mental disorders.
The largest global PAF was observed for childhood adversities, which accounted for about two-fifths (38%) of global cases of schizophrenia spectrum disorders. This finding is not surprising, given the relatively high prevalence of adverse childhood experiences, including “toxic stressors”, which can range from bullying experiences to physical or sexual abuse, neglect and even to war crimes [58]. These findings align with psychodynamic theories [59, 60] as well with the more recent social defeat model [61]. Furthermore, a dose-response relationship between childhood adversities and psychotic disorders has been observed [62]. Neurobiologically, childhood adversities are associated with sensitisation of dopamine neurotransmission [63], the key neurotransmitters in psychotic disorders. This study is also the first one to quantify the preventive potential of the clinical high-risk state for psychosis as 12% of global cases of psychosis. As the clinical high risk state for psychosis paradigm was primarily conceived as a targeted and not public health approach, its smaller PAF compared to childhood adversity is expected [3]. Interestingly, the global preventive capacity of other established risk factors, such as cannabis use (10%), was also smaller than childhood adversities and of comparable magnitude as the clinical high-risk state for psychosis. These findings temper recent controversies juxtaposing the utility of preventing psychosis by targeting cannabis abuse or the clinical high-risk state for psychosis, suggesting that both targets hold similar preventive capacity. Notably, as for any other factors in the current study, there was no assumption that these two factors (and therefore PAFs) are independent (e.g., 26% of individuals at clinical high risk for psychosis are also current cannabis users);[64] their combined preventive capacity needs further elucidation by future research [4, 9].
The second-largest PAF was observed for tobacco smoking and opioid use disorder (25%), a finding broadly consistent with established associations between nicotine and opioid dependence [65, 66] and with shared biological underpinnings, extended reinforcement, and cross-tolerance [67]. The third-largest PAF was observed for job strain and depression (18%), which emerges as a core modifiable target among working adults [68]. This result aligns with the substantial and widespread impact of job strain on other physical health outcomes, including metabolic syndrome [69], coronary heart disease [70], diabetes [71], stroke [72], musculoskeletal pain [73] and even mortality [74].
Interestingly, one specific type of childhood adversity (sexual abuse) emerged also as a preventive target for depressive disorders (13%), highlighting its potential transdiagnostic capacity, which could allow preventing multiple mental disorders, and better justify the cost of any preventive intervention.
Overall, the public health implication of these findings is to recommend prioritising resources to reduce a small risk among many (and ideally across different mental disorders) rather than vice versa [75]. This approach could be further enhanced by simultaneously targeting mental and physical health domains, maximising the resulting preventive potential. For example, we identified three preventive targets pertaining to physical health domains (PAFs from 10-25%), which have been associated with neuroinflammatory mechanisms [76]: three metabolic factors and depression, insufficient physical activity and Alzheimer’s disease, tobacco smoking and opioid use disorders.
Notably, while we primarily focused on global PAFs, the prevalence of these factors varies profoundly across different countries and demographic groups. To explore this issue, we performed sensitivity analyses using specific prevalence data. The largest PAF was confirmed for childhood adversities, with comparable magnitude across high-, middle- and low-income countries. Country-level PAFs were highly variable for tobacco smoking, job strain, and cannabis use. The PAF for tobacco smoking and opioid use disorders was higher in Europe versus the USA and in men versus women, while the PAF for insufficient physical activity and Alzheimer’s disease tended to be reduced in low-income countries. Some PAFs were particularly marked in specific groups: childhood sexual abuse and depressive disorders among women, four or five metabolic risk factors and depressive disorders in adults >70 years, T2DM and vascular dementia or Alzheimer’s disease in adults >70 years. This great variation of PAFs may indicate a complex interplay of sociodemographic, health, and economic factors, which future research should better address.
Although our results for factors with the largest PAFs are derived from high- to medium-quality meta-analyses (with few exceptions), this study has some important limitations. First, while the term “attributable” in the PAF usually has a causal interpretation [77], there are no clear aetiopathological factors identified for mental disorders but only statistical associations. As most associations of risk factors with mental disorders typically emerged from observational cohort studies, which are liable to confounding [30], the estimated PAF effect is not adjusted for all possible confounders (and the risk factors are not necessarily independent and probably intercorrelated, see eLimitation). However, we did carefully identify potential confounding by indication. Because of these limitations the PAFs reported in this study should be distinguished from the aetiologic fraction [78]. Another limitation is that there are no established cut-offs to distinguish between large and small PAFs. Furthermore, the PAF is a static measure that assumes that removing an exposure does not affect the person–time at risk and onwards effects, which may not be true for some factors [30], particularly for those exerting their effect during early neurodevelopmental stages. A transmission PAF (tPAF) [79, 80] has been suggested to mathematically estimate onward transmission of the potential long-term preventive gains [75]. Possible caveats that may result in over- or underestimation of the prevalence estimates for several risk factors should also be considered. Reliable population-level data were not always available, and we were unable to calculate the global PAF for some factors (low frequency of social contacts, sexual dysfunction, sleep disturbances) and the specific PAFs for several factors. Future research should address the global and specific prevalence of these factors. Finally, while this study focuses on the hypothetical preventive capacity of robust non-primarily genetic targets; the real-world effectiveness of specific preventive interventions targeting these factors should be demonstrated and appraised by separate analyses.
Conclusions
Addressing several potentially modifiable risk factors, in particular childhood adversities, can potentially reduce the global population-level incidence of mental disorders. Future research should prioritise these preventive targets.
Data availability
The meta-analytic data are published and freely accessible.
References
Solmi M, Radua J, Olivola M, Croce E, Soardo L, Salazar de Pablo G, et al. Age at onset of mental disorders worldwide: large-scale meta-analysis of 192 epidemiological studies. Mol Psychiatry. 2022;27:281–95.
Arango C, Diaz-Caneja CM, McGorry PD, Rapoport J, Sommer IE, Vorstman JA, et al. Preventive strategies for mental health. Lancet Psychiatry. 2018;5:591–604.
Fusar-Poli P, Correll CU, Arango C, Berk M, Patel V, Ioannidis JPA. Preventive psychiatry: a blueprint for improving the mental health of young people. World Psychiatry. 2021;20:200–21.
Fusar-Poli P, Salazar de Pablo G, Correll CU, Meyer-Lindenberg A, Millan MJ, Borgwardt S, et al. Prevention of psychosis: advances in detection, prognosis, and intervention. JAMA Psychiatry. 2020;77:755–65.
Fusar-Poli P. The clinical high-risk state for psychosis (CHR-P), version II. Schizophr Bull. 2017;43:44–47.
Salazar de Pablo G, Catalan A, Fusar-Poli P. Clinical validity of DSM-5 attenuated psychosis syndrome: advances in diagnosis, prognosis, and treatment. JAMA Psychiatry. 2020;77:311–20.
Salazar de Pablo G, De Micheli A, Nieman DH, Correll CU, Kessing LV, Pfennig A, et al. Universal and selective interventions to promote good mental health in young people: Systematic review and meta-analysis. Eur Neuropsychopharmacol. 2020;41:28–39.
Fusar-Poli P, Bauer M, Borgwardt S, Bechdolf A, Correll CU, Do KQ, et al. European college of neuropsychopharmacology network on the prevention of mental disorders and mental health promotion (ECNP PMD-MHP). Eur Neuropsychopharmacol. 2019;29:1301–11.
Catalan A, Salazar de Pablo G, Vaquerizo Serrano J, Mosillo P, Baldwin H, Fernandez-Rivas A, et al. Annual research review: prevention of psychosis in adolescents—systematic review and meta-analysis of advances in detection, prognosis and intervention. J Child Psychol Psychiatry. 2021;62:657–73.
Clark EG. Natural history of syphilis and levels of prevention. Br J Vener Dis. 1954;30:191–7.
Wray NR, Lin T, Austin J, McGrath JJ, Hickie IB, Murray GK, et al. From basic science to clinical application of polygenic risk scores: a primer. JAMA Psychiatry. 2021;78:101–9.
Trubetskoy V, Pardiñas AF, Qi T, Panagiotaropoulou G, Awasthi S, Bigdeli TB, et al.Schizophrenia Working Group of the Psychiatric Genomics Consortium. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature. 2022;604:502–8.
Marsman A, Pries LK, Ten Have M, de Graaf R, van Dorsselaer S, Bak M, et al. Do current measures of polygenic risk for mental disorders contribute to population variance in mental health? Schizophr Bull. 2020;46:1353–62.
Uher R, Zwicker A. Etiology in psychiatry: embracing the reality of poly-gene-environmental causation of mental illness. World Psychiatry. 2017;16:121–9.
Fusar-Poli P, Radua J. Ten simple rules for conducting umbrella reviews. Evid Based Ment Health. 2018;21:95–100.
Ioannidis JP. Integration of evidence from multiple meta-analyses: a primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. Cmaj. 2009;181:488–93.
Radua J, Ramella-Cravaro V, Ioannidis JPA, Reichenberg A, Phiphopthatsanee N, Amir T, et al. What causes psychosis? An umbrella review of risk and protective factors. World Psychiatry. 2018;17:49–66.
Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Health. 2015;13:132–40.
Bellou V, Belbasis L, Tzoulaki I, Evangelou E, Ioannidis JP. Environmental risk factors and Parkinson’s disease: an umbrella review of meta-analyses. Parkinsonism Relat Disord. 2016;23:1–9.
Belbasis L, Bellou V, Evangelou E, Tzoulaki I. Environmental factors and risk of multiple sclerosis: findings from meta-analyses and Mendelian randomization studies. Mult Scler. 2020;26:397–404.
Belbasis L, Bellou V, Evangelou E, Ioannidis JP, Tzoulaki I. Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neurol. 2015;14:263–73.
Dragioti E, Solmi M, Favaro A, Fusar-Poli P, Dazzan P, Thompson T, et al. Association of antidepressant use with adverse health outcomes: a systematic umbrella review. JAMA Psychiatry. 2019;76:1241–55.
Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014;348:g2035.
Fusar-Poli P, Hijazi Z, Stahl D, Steyerberg EW. The science of prognosis in psychiatry: a review. JAMA Psychiatry. 2018;75:1289–97.
Bray F, Soerjomataram I. Population attributable fractions continue to unmask the power of prevention. Br J Cancer. 2018;118:1031–2.
Shield KD, Parkin DM, Whiteman DC, Rehm J, Viallon V, Micallef CM, et al. Population attributable and preventable fractions: cancer risk factor surveillance, and cancer policy projection. Curr Epidemiol Rep. 2016;3:201–11.
Whiteman DC, Webb PM, Green AC, Neale RE, Fritschi L, Bain CJ, et al. Cancers in Australia in 2010 attributable to modifiable factors: summary and conclusions. Aust N. Z J Public Health. 2015;39:477–84.
Rezende LF, Eluf-Neto J. Population attributable fraction: planning of diseases prevention actions in Brazil. Rev Saude Publica 2016;50:30.
Mukadam N, Sommerlad A, Huntley J, Livingston G. Population attributable fractions for risk factors for dementia in low-income and middle-income countries: an analysis using cross-sectional survey data. Lancet Glob Health. 2019;7:e596–e603.
Mansournia MA, Altman DG. Population attributable fraction. BMJ. 2018;360:k757.
Radua J. PRISMA 2020—an updated checklist for systematic reviews and meta-analyses. Neurosci Biobehav Rev. 2021;124:324–5.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Mentis AA, Dardiotis E, Efthymiou V, Chrousos GP. Non-genetic risk and protective factors and biomarkers for neurological disorders: a meta-umbrella systematic review of umbrella reviews. BMC Med. 2021;19:6.
Rasic D, Hajek T, Alda M, Uher R. Risk of mental illness in offspring of parents with schizophrenia, bipolar disorder, and major depressive disorder: a meta-analysis of family high-risk studies. Schizophr Bull. 2014;40:28–38.
Pepper EJ, Pathmanathan S, McIlrae S, Rehman FU, Cardno AG. Associations between risk factors for schizophrenia and concordance in four monozygotic twin samples. Am J Med Genet B Neuropsychiatr Genet. 2018;177:503–10.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6.
Bellou V, Belbasis L, Tzoulaki I, Middleton LT, Ioannidis JPA, Evangelou E. Systematic evaluation of the associations between environmental risk factors and dementia: an umbrella review of systematic reviews and meta-analyses. Alzheimers Dement. 2017;13:406–18.
Solmi M, Kohler CA, Stubbs B, Koyanagi A, Bortolato B, Monaco F, et al. Environmental risk factors and nonpharmacological and nonsurgical interventions for obesity: An umbrella review of meta-analyses of cohort studies and randomized controlled trials. Eur J Clin Invest. 2018;48:e12982.
Kyriacou DN, Lewis RJ. Confounding by indication in clinical research. J Am Med Assoc. 2016;316:1818–9.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Collaborators GBDO, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl J Med. 2017;377:13–27.
Degenhardt L, Ferrari AJ, Calabria B, Hall WD, Norman RE, McGrath J, et al. The global epidemiology and contribution of cannabis use and dependence to the global burden of disease: results from the GBD 2010 study. PLoS ONE. 2013;8:e76635.
Reitsma MB, Flor LS, Mullany EC, Gupta V, Hay SI, Gakidou E. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and initiation among young people in 204 countries and territories, 1990–2019. The Lancet Public Health. 2021;6:e472–81.
Santomauro DF, Melen S, Mitchison D, Vos T, Whiteford H, Ferrari AJ. The hidden burden of eating disorders: an extension of estimates from the Global Burden of Disease Study 2019. Lancet Psychiatry. 2021;8:320–8.
Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–22.
Obesity WHOCo, World Health O. Obesity: preventing and managing the global epidemic: report of a WHO consultation. Geneva: World Health Organization; 2000.
Munn Z, Moola S, Riitano D, Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag. 2014;3:123–8.
Levin ML. The occurrence of lung cancer in man. Acta Unio Int Contra Cancrum. 1953;9:531–41.
Lin C-K, Chen S-T. Estimation and application of population attributable fraction in ecological studies. Environmental Health. 2019;18:1–3.
Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. J Am Med Assoc. 1998;280:1690–1.
Daly LE. Confidence limits made easy: interval estimation using a substitution method. Am J Epidemiol. 1998;147:783–90.
Morgenstern H, Bursic ES. A method for using epidemiologic data to estimate the potential impact of an intervention on the health status of a target population. J Community Health. 1982;7:292–309.
Belbasis L, Kohler CA, Stefanis N, Stubbs B, van Os J, Vieta E, et al. Risk factors and peripheral biomarkers for schizophrenia spectrum disorders: an umbrella review of meta-analyses. Acta Psychiatr Scand. 2018;137:88–97.
Kohler CA, Evangelou E, Stubbs B, Solmi M, Veronese N, Belbasis L, et al. Mapping risk factors for depression across the lifespan: an umbrella review of evidence from meta-analyses and Mendelian randomization studies. J Psychiatr Res. 2018;103:189–207.
Kim JY, Son MJ, Son CY, Radua J, Eisenhut M, Gressier F, et al. Environmental risk factors and biomarkers for autism spectrum disorder: an umbrella review of the evidence. Lancet Psychiatry. 2019;6:590–600.
Kim JH, Kim JY, Lee J, Jeong GH, Lee E, Lee S, et al. Environmental risk factors, protective factors, and peripheral biomarkers for ADHD: an umbrella review. Lancet Psychiatry. 2020;7:955–70.
Solmi M, Dragioti E, Croatto G, Radua J, Borgwardt S, Carvalho AF, et al. Risk and protective factors for cannabis, cocaine, and opioid use disorders: an umbrella review of meta-analyses of observational studies. Neurosci Biobehav Rev. 2021;126:243–51.
Nelson CA, Scott RD, Bhutta ZA, Harris NB, Danese A, Samara M. Adversity in childhood is linked to mental and physical health throughout life. BMJ. 2020;371:m3048.
Kirshner LA. Trauma and psychosis: a review and framework for psychoanalytic understanding. Int Forum Psychoanalysis. 2013;24:216–24.
Coughlan H, Cannon M. Does childhood trauma play a role in the aetiology of psychosis? A review of recent evidence. BJPsych Adv. 2018;23:307–15.
Rosenfield PJ, Jiang D, Pauselli L. Childhood adversity and psychotic disorders: epidemiological evidence, theoretical models and clinical considerations. Schizophr Res. 2021;S0920-9964:00217–6.
McKay MT, Cannon M, Chambers D, Conroy RM, Coughlan H, Dodd P, et al. Childhood trauma and adult mental disorder: A systematic review and meta-analysis of longitudinal cohort studies. Acta Psychiatr Scand. 2021;143:189–205.
Egerton A, Valmaggia LR, Howes OD, Day F, Chaddock CA, Allen P, et al. Adversity in childhood linked to elevated striatal dopamine function in adulthood. Schizophr Res. 2016;176:171–6.
Farris MS, Shakeel MK, Addington J. Cannabis use in individuals at clinical high-risk for psychosis: a comprehensive review. Soc Psychiatry Psychiatr Epidemiol. 2020;55:527–37.
Rajabi A, Dehghani M, Shojaei A, Farjam M, Motevalian SA. Association between tobacco smoking and opioid use: a meta-analysis. Addict Behav. 2019;92:225–35.
Parker MA, Streck JM, Sigmon SC. Associations between opioid and nicotine dependence in nationally representative samples of United States adult daily smokers. Drug Alcohol Depend. 2018;186:167–70.
Kohut SJ. Interactions between nicotine and drugs of abuse: a review of preclinical findings. Am J Drug Alcohol Abus. 2017;43:155–70.
Madsen IEH, Nyberg ST, Magnusson Hanson LL, Ferrie JE, Ahola K, Alfredsson L, et al. Job strain as a risk factor for clinical depression: systematic review and meta-analysis with additional individual participant data. Psychol Med. 2017;47:1342–56.
Watanabe K, Sakuraya A, Kawakami N, Imamura K, Ando E, Asai Y, et al. Work-related psychosocial factors and metabolic syndrome onset among workers: a systematic review and meta-analysis. Obes Rev. 2018;19:1557–68.
Dragano N, Siegrist J, Nyberg ST, Lunau T, Fransson EI, Alfredsson L, et al. Effort-reward imbalance at work and incident coronary heart disease: a multicohort study of 90,164 individuals. Epidemiology. 2017;28:619–26.
Sui H, Sun N, Zhan L, Lu X, Chen T, Mao X. Association between work-related stress and risk for type 2 diabetes: a systematic review and meta-analysis of prospective cohort studies. PLoS ONE. 2016;11:e0159978.
Fransson EI, Nyberg ST, Heikkila K, Alfredsson L, Bjorner JB, Borritz M, et al. Job strain and the risk of stroke: an individual-participant data meta-analysis. Stroke. 2015;46:557–9.
Amiri S, Behnezhad S. Is job strain a risk factor for musculoskeletal pain? A systematic review and meta-analysis of 21 longitudinal studies. Public Health. 2020;181:158–67.
Amiri S, Behnezhad S. Job strain and mortality ratio: a systematic review and meta-analysis of cohort studies. Public Health. 2020;181:24–33.
Mishra S, Baral SD. Rethinking the population attributable fraction for infectious diseases. Lancet Infect Dis. 2020;20:155–7.
Hanc T, Cortese S. Attention deficit/hyperactivity-disorder and obesity: a review and model of current hypotheses explaining their comorbidity. Neurosci Biobehav Rev. 2018;92:16–28.
Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3rd edn. Philadelphia: Wolters Kluwer Health; 2015, p 1 online resource (768 p.).
Greenland S. Concepts and pitfalls in measuring and interpreting attributable fractions, prevented fractions, and causation probabilities. Ann Epidemiol. 2015;25:155–61.
Mishra S, Pickles M, Blanchard JF, Moses S, Boily MC. Distinguishing sources of HIV transmission from the distribution of newly acquired HIV infections: why is it important for HIV prevention planning? Sex Transm Infect. 2014;90:19–25.
Mishra S, Boily MC, Schwartz S, Beyrer C, Blanchard JF, Moses S, et al. Data and methods to characterize the role of sex work and to inform sex work programs in generalized HIV epidemics: evidence to challenge assumptions. Ann Epidemiol. 2016;26:557–69.
Acknowledgements
The study was funded by a Wellcome Trust grant to Paolo Fusar-Poli (Early DetectioN of menTal disorERs, ENTER: 215793/Z/19/Z). Dr Arango receives support from the Spanish Ministry of Science and Innovation. Instituto de Salud Carlos III (SAM16PE07CP1, PI16/02012, PI19/024), co-financed by ERDF Funds from the European Commission, “A way of making Europe”, CIBERSAM. Madrid Regional Government (B2017/BMD-3740 AGES-CM-2), European Union Structural Funds. European Union Seventh Framework Program under grant agreements FP7-4-HEALTH-2009-2.2.1-2-241909 (Project EU-GEI), FP7- HEALTH-2013-2.2.1-2-603196 (Project PSYSCAN) and FP7- HEALTH-2013-2.2.1-2-602478 (Project METSY); and European Union H2020 Program under the Innovative Medicines Initiative 2 Joint Undertaking (grant agreement No 115916, Project PRISM, and grant agreement No 777394, Project AIMS-2-TRIALS), Fundación Familia Alonso and Fundación Alicia Koplowitz.
Author information
Authors and Affiliations
Contributions
PFP conceived the study, ED and JQ conducted the analyses, all authors provided substantial conceptual contributions and approved the final version of the study.
Corresponding author
Ethics declarations
Competing interests
Dr. Arango has been a consultant to or has received honoraria or grants from Acadia, Angelini, Boehringer, Gedeon Richter, Janssen Cilag, Lundbeck, Minerva, Otsuka, Pfizer, Roche, Sage, Servier, Shire, Schering Plough, Sumitomo Dainippon Pharma, Sunovion and Takeda outside the current work. Paolo Fusar-Poli has received research or personal fees from Lundbeck, Angelini, Menarini and Boehringer Ingelheim outside the current work. Dr. Correll has been a consultant and/or advisor to or has received honoraria from AbbVie, Acadia, Alkermes, Allergan, Angelini, Aristo, Axsome, Damitsa, Gedeon Richter, Hikma, IntraCellular Therapies, Janssen/J&J, Karuna, LB Pharma, Lundbeck, MedAvante-ProPhase, MedInCell, Medscape, Merck, Mitsubishi Tanabe Pharma, Mylan, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Servier, SK Life Science, Sumitomo Dainippon, Sunovion, Supernus, Takeda, Teva, and Viatris. He provided expert testimony for Janssen and Otsuka. He served on a Data Safety Monitoring Board for Lundbeck, Rovi, Supernus, and Teva. He has received grant support from Janssen and Takeda. He received royalties from UpToDate and is also a stock option holder of LB Pharma. Dr. Jones is funded by the NIHR ARC East of England. The remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dragioti, E., Radua, J., Solmi, M. et al. Global population attributable fraction of potentially modifiable risk factors for mental disorders: a meta-umbrella systematic review. Mol Psychiatry 27, 3510–3519 (2022). https://doi.org/10.1038/s41380-022-01586-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-022-01586-8
This article is cited by
-
Body weight has varying effects on the risk of neurological and psychiatric disorders
Nature Mental Health (2024)
-
Predicting Onset of Visual Hallucinations Using Pareidolias: A Qualitative Exploration of the Ethics of a Digital App to Detect a Possible Biomarker
Journal of Technology in Behavioral Science (2024)
-
Risk and Protective Factors of Self-harm and Suicidality in Adolescents: An Umbrella Review with Meta-Analysis
Journal of Youth and Adolescence (2024)
-
Incidence, prevalence, and global burden of schizophrenia - data, with critical appraisal, from the Global Burden of Disease (GBD) 2019
Molecular Psychiatry (2023)
-
Association between non-medical cannabis legalization and emergency department visits for cannabis-induced psychosis
Molecular Psychiatry (2023)