Abstract
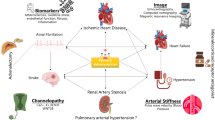
Primary aldosteronism (PA), the most common cause of secondary hypertension, is a well-recognized condition that can lead to cardiovascular and renal complications. PA is frequently left undiagnosed and untreated, leading to aldosterone-specific morbidity and mortality. In this review we highlight the evidence linking PA with other conditions such as (i) diabetes mellitus, (ii) obstructive sleep apnea, and (iii) bone health, along with clinical implications and proposed underlying mechanisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Young WF Jr., Calhoun DA, Lenders JWM, Stowasser M, Textor SC. Screening for endocrine hypertension: an endocrine society scientific statement. Endocr Rev. 2017;38:103–22.
Sukor N. Endocrine hypertension–current understanding and comprehensive management review. Eur J Intern Med. 2011;22:433–40.
Stowasser M, Taylor PJ, Pimenta E, Ahmed AH, Gordon RD. Laboratory investigation of primary aldosteronism. Clin Biochemist Rev. 2010;31:39–56.
Sukor N. Primary aldosteronism: from bench to bedside. Endocrine. 2012;41:31–9.
Conn JW, Cohen EL, Rovner DR, Nesbit RM. Normokalemic primary aldosteronism. A detectable cause of curable "essential" hypertension. J Am Med Assoc. 1965;193:200–6.
Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889–916.
Huang KH, Yu CC, Hu YH, Chang CC, Chan CK, Liao SC, et al. Targeted treatment of primary aldosteronism - The consensus of Taiwan Society of Aldosteronism. J Formos Med Assoc. 2019;118:72–82.
Sukor N, Gordon RD, Ku YK, Jones M, Stowasser M. Role of unilateral adrenalectomy in bilateral primary aldosteronism: a 22-year single center experience. J Clin Endocrinol Metab. 2009;94:2437–45.
Young WF Jr. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives. J Intern Med. 2019;285:126–48.
Sukor N. Secondary hypertension: a condition not to be missed. Postgrad Med J. 2011;87:706–13.
Reincke M, Fischer E, Gerum S, Merkle K, Schulz S, Pallauf A, et al. Observational study mortality in treated primary aldosteronism: the German Conn's registry. Hypertension. 2012;60:618–24.
Catena C, Colussi G, Nadalini E, Chiuch A, Baroselli S, Lapenna R, et al. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch Intern Med. 2008;168:80–5.
Sukor N. Clinical approach to young hypertension. Brunei Int. Medl J. 2013;9:81–92.
Dominguez A, Gupta S. Hyperaldosteronism. Treasure Island, FL: StatPearls; 2019.
Kline GA, Prebtani APH, Leung AA, Schiffrin EL. Primary aldosteronism: a common cause of resistant hypertension. CMAJ. 2017;189:E773–E8.
Dudenbostel T, Calhoun DA. Resistant hypertension, obstructive sleep apnoea and aldosterone. J Hum Hypertens. 2012;26:281–7.
Jin ZN, Wei YX. Meta-analysis of effects of obstructive sleep apnea on the renin-angiotensin-aldosterone system. J Geriatr Cardiol. 2016;13:333–43.
Yang SJ, Jiang XT, Zhang XB, Yin XW, Deng WX. Does continuous positive airway pressure reduce aldosterone levels in patients with obstructive sleep apnea? Sleep Breath. 2016;20:921–8.
Salcuni AS, Carnevale V, Battista C, Palmieri S, Eller-Vainicher C, Guarnieri V, et al. Primary aldosteronism as a cause of secondary osteoporosis. Eur J Endocrinol. 2017;177:431–7.
Arlt W, Lang K, Sitch AJ, Dietz AS, Rhayem Y, Bancos I, et al. Steroid metabolome analysis reveals prevalent glucocorticoid excess in primary aldosteronism. JCI Insight. 2017;2.
Fallo F, Veglio F, Bertello C, Sonino N, Della Mea P, Ermani M, et al. Prevalence and characteristics of the metabolic syndrome in primary aldosteronism. J Clin Endocrinol Metab. 2006;91:454–9.
Mosso LM, Carvajal CA, Maiz A, Ortiz EH, Castillo CR, Artigas RA, et al. A possible association between primary aldosteronism and a lower beta-cell function. J Hypertens. 2007;25:2125–30.
Reincke M, Meisinger C, Holle R, Quinkler M, Hahner S, Beuschlein F, et al. Is primary aldosteronism associated with diabetes mellitus? Results of the German Conn's Registry. Horm Metab Res. 2010;42:435–9.
Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6:51–9.
Hanslik G, Wallaschofski H, Dietz A, Riester A, Reincke M, Allolio B, et al. Increased prevalence of diabetes mellitus and the metabolic syndrome in patients with primary aldosteronism of the German Conn's Registry. Eur J Endocrinol. 2015;173:665–75.
Akehi Y, Yanase T, Motonaga R, Umakoshi H, Tsuiki M, Takeda Y, et al. High prevalence of diabetes in patients with primary aldosteronism (PA) associated with subclinical hypercortisolism and prediabetes more prevalent in bilateral than unilateral PA: a Large, Multicenter Cohort Study in Japan. Diabetes Care. 2019;42:938–45.
Matrozova J, Steichen O, Amar L, Zacharieva S, Jeunemaitre X, Plouin PF. Fasting plasma glucose and serum lipids in patients with primary aldosteronism: a controlled cross-sectional study. Hypertension. 2009;53:605–10.
Widimsky J Jr., Sindelka G, Haas T, Prazny M, Hilgertova J, Skrha J. Impaired insulin action in primary hyperaldosteronism. Physiological Res. 2000;49:241–4.
Ishimori M, Takeda N, Okumura S, Murai T, Inouye H, Yasuda K. Increased insulin sensitivity in patients with aldosterone producing adenoma. Clin Endocrinol. 1994;41:433–8.
Tsurutani Y, Sugisawa C, Ishida A, Inoue K, Saito J, Omura M, et al. Aldosterone excess may inhibit insulin secretion: A comparative study on glucose metabolism pre- and post-adrenalectomy in patients with primary aldosteronism. Endocr J. 2017;64:339–46.
Sindelka G, Widimsky J, Haas T, Prazny M, Hilgertova J, Skrha J. Insulin action in primary hyperaldosteronism before and after surgical or pharmacological treatment. Exp Clin Endocrinol Diabetes. 2000;108:21–5.
Fischer E, Adolf C, Pallauf A, Then C, Bidlingmaier M, Beuschlein F, et al. Aldosterone excess impairs first phase insulin secretion in primary aldosteronism. J Clin Endocrinol Metab. 2013;98:2513–20.
Catena C, Lapenna R, Baroselli S, Nadalini E, Colussi G, Novello M, et al. Insulin sensitivity in patients with primary aldosteronism: a follow-up study. J Clin Endocrinol Metab. 2006;91:3457–63.
Strauch B, Widimsky J, Sindelka G, Skrha J. Does the treatment of primary hyperaldosteronism influence glucose tolerance? Physiological Res. 2003;52:503–6.
Giacchetti G, Ronconi V, Turchi F, Agostinelli L, Mantero F, Rilli S, et al. Aldosterone as a key mediator of the cardiometabolic syndrome in primary aldosteronism: an observational study. J Hypertens. 2007;25:177–86.
Wu VC, Chueh SJ, Chen L, Chang CH, Hu YH, Lin YH, et al. Risk of new-onset diabetes mellitus in primary aldosteronism: a population study over 5 years. J Hypertens. 2017;35:1698–708.
Chen ZW, Hung CS, Wu VC, Lin YH, group Ts. Primary aldosteronism and cerebrovascular diseases. Endocrinol Metab. 2018;33:429–34.
Monticone S, D'Ascenzo F, Moretti C, Williams TA, Veglio F, Gaita F, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018;6:41–50.
Kreze A Sr., Kreze-Spirova E, Mikulecky M. Diabetes mellitus in primary aldosteronism. Bratisl Lekarske Listy. 2000;101:187–90.
Fallo F, Della Mea P, Sonino N, Bertello C, Ermani M, Vettor R, et al. Adiponectin and insulin sensitivity in primary aldosteronism. Am J Hypertens. 2007;20:855–61.
Zennaro MC, Caprio M, Feve B. Mineralocorticoid receptors in the metabolic syndrome. Trends Endocrinol Metab. 2009;20:444–51.
Chanut F. Potassium channels rule over insulin release with an ion fist. PLoS Biol. 2006;4:e53.
Gommers LM, Hoenderop JG, Bindels RJ, de Baaij JH. Hypomagnesemia in type 2 diabetes: a vicious circle? Diabetes. 2016;65:3–13.
Barr CS, Lang CC, Hanson J, Arnott M, Kennedy N, Struthers AD. Effects of adding spironolactone to an angiotensin-converting enzyme inhibitor in chronic congestive heart failure secondary to coronary artery disease. Am J Cardiol. 1995;76:1259–65.
Gao X, Peng L, Adhikari CM, Lin J, Zuo Z. Spironolactone reduced arrhythmia and maintained magnesium homeostasis in patients with congestive heart failure. J Card Fail. 2007;13:170–7.
Tauchmanova L, Rossi R, Biondi B, Pulcrano M, Nuzzo V, Palmieri EA, et al. Patients with subclinical Cushing's syndrome due to adrenal adenoma have increased cardiovascular risk. J Clin Endocrinol Metab. 2002;87:4872–8.
Gerards J, Heinrich DA, Adolf C, Meisinger C, Rathmann W, Sturm L, et al. Impaired glucose metabolism in primary aldosteronism is associated with cortisol co-secretion. J Clin Endocrinol Metab. 2019;104:3192–202.
Di Dalmazi G, Vicennati V, Garelli S, Casadio E, Rinaldi E, Giampalma E, et al. Cardiovascular events and mortality in patients with adrenal incidentalomas that are either non-secreting or associated with intermediate phenotype or subclinical Cushing's syndrome: a 15-year retrospective study. Lancet Diabetes Endocrinol. 2014;2:396–405.
Luther JM. Effects of aldosterone on insulin sensitivity and secretion. Steroids. 2014;91:54–60.
Selvaraj J, Muthusamy T, Srinivasan C, Balasubramanian K. Impact of excess aldosterone on glucose homeostasis in adult male rat. Clin Chim Acta. 2009;407:51–7.
Yamashita R, Kikuchi T, Mori Y, Aoki K, Kaburagi Y, Yasuda K, et al. Aldosterone stimulates gene expression of hepatic gluconeogenic enzymes through the glucocorticoid receptor in a manner independent of the protein kinase B cascade. Endocr J. 2004;51:243–51.
Campion J, Lahera V, Cachofeiro V, Maestro B, Davila N, Carranza MC, et al. In vivo tissue specific modulation of rat insulin receptor gene expression in an experimental model of mineralocorticoid excess. Mol Cell Biochem. 1998;185:177–82.
Hitomi H, Kiyomoto H, Nishiyama A, Hara T, Moriwaki K, Kaifu K, et al. Aldosterone suppresses insulin signaling via the downregulation of insulin receptor substrate-1 in vascular smooth muscle cells. Hypertension. 2007;50:750–5.
Cascella T, Radhakrishnan Y, Maile LA, Busby WH Jr., Gollahon K, Colao A, et al. Aldosterone enhances IGF-I-mediated signaling and biological function in vascular smooth muscle cells. Endocrinology. 2010;151:5851–64.
Schneider HJ, Friedrich N, Klotsche J, Schipf S, Nauck M, Volzke H, et al. Prediction of incident diabetes mellitus by baseline IGF1 levels. Eur J Endocrinol. 2011;164:223–9.
Calhoun DA, Nishizaka MK, Zaman MA, Harding SM. Aldosterone excretion among subjects with resistant hypertension and symptoms of sleep apnea. Chest. 2004;125:112–7.
Gonzaga CC, Gaddam KK, Ahmed MI, Pimenta E, Thomas SJ, Harding SM, et al. Severity of obstructive sleep apnea is related to aldosterone status in subjects with resistant hypertension. J Clin Sleep Med. 2010;6:363–8.
Pratt-Ubunama MN, Nishizaka MK, Boedefeld RL, Cofield SS, Harding SM, Calhoun DA. Plasma aldosterone is related to severity of obstructive sleep apnea in subjects with resistant hypertension. Chest. 2007;131:453–9.
Ke X, Guo W, Peng H, Hu C, Zhang H, Peng C, et al. Association of aldosterone excess and apnea-hypopnea index in patients with resistant hypertension. Sci Rep. 2017;7:45241.
Muxfeldt ES, Margallo VS, Guimaraes GM, Salles GF. Prevalence and associated factors of obstructive sleep apnea in patients with resistant hypertension. Am J Hypertens. 2014;27:1069–78.
Barcelo A, Pierola J, Esquinas C, de la Pena M, Arque M, Alonso-Fernandez A, et al. Relationship between aldosterone and the metabolic syndrome in patients with obstructive sleep apnea hypopnea syndrome: effect of continuous positive airway pressure treatment. PLoS ONE. 2014;9:e84362.
Di Murro A, Petramala L, Cotesta D, Zinnamosca L, Crescenzi E, Marinelli C, et al. Renin-angiotensin-aldosterone system in patients with sleep apnoea: prevalence of primary aldosteronism. J Renin Angiotensin Aldosterone Syst. 2010;11:165–72.
Svatikova A, Olson LJ, Wolk R, Phillips BG, Adachi T, Schwartz GL, et al. Obstructive sleep apnea and aldosterone. Sleep. 2009;32:1589–92.
Lykouras D, Theodoropoulos K, Sampsonas F, Lagiou O, Lykouras M, Spiropoulou A, et al. The impact of obstructive sleep apnea syndrome on renin and aldosterone. Eur Rev Med Pharmacol Sci. 2015;19:4164–70.
Gaddam K, Pimenta E, Thomas SJ, Cofield SS, Oparil S, Harding SM, et al. Spironolactone reduces severity of obstructive sleep apnoea in patients with resistant hypertension: a preliminary report. J Hum Hypertens. 2010;24:532–7.
Yang L, Zhang H, Cai M, Zou Y, Jiang X, Song L, et al. Effect of spironolactone on patients with resistant hypertension and obstructive sleep apnea. Clin Exp Hypertens. 2016;38:464–8.
Krasinska B, Miazga A, Cofta S, Szczepaniak-Chichel L, Trafas T, Krasinski Z, et al. Effect of eplerenone on the severity of obstructive sleep apnea and arterial stiffness in patients with resistant arterial hypertension. Polskie Archiwum Med Wewnetrznej. 2016;126:330–9.
Wang E, Chomsky-Higgins K, Chen Y, Nwaogu I, Seib CD, Shen WT, et al. Treatment of primary aldosteronism reduces the probability of obstructive sleep apnea. J Surgical Res. 2019;236:37–43.
Wolley MJ, Pimenta E, Calhoun D, Gordon RD, Cowley D, Stowasser M. Treatment of primary aldosteronism is associated with a reduction in the severity of obstructive sleep apnoea. J Hum Hypertens. 2017;31:561–7.
Saarelainen S, Hasan J, Siitonen S, Seppala E. Effect of nasal CPAP treatment on plasma volume, aldosterone and 24-h blood pressure in obstructive sleep apnoea. J Sleep Res. 1996;5:181–5.
Moller DS, Lind P, Strunge B, Pedersen EB. Abnormal vasoactive hormones and 24-hour blood pressure in obstructive sleep apnea. Am J Hypertens. 2003;16:274–80.
Nicholl DD, Hanly PJ, Poulin MJ, Handley GB, Hemmelgarn BR, Sola DY, et al. Evaluation of continuous positive airway pressure therapy on renin-angiotensin system activity in obstructive sleep apnea. Am J Respiratory Crit Care Med. 2014;190:572–80.
Pimenta E, Calhoun DA, Oparil S. Sleep apnea, aldosterone, and resistant hypertension. Prog Cardiovascular Dis. 2009;51:371–80.
Kasai T, Bradley TD, Friedman O, Logan AG. Effect of intensified diuretic therapy on overnight rostral fluid shift and obstructive sleep apnoea in patients with uncontrolled hypertension. J Hypertens. 2014;32:673–80.
Lavie L, Lavie P. Molecular mechanisms of cardiovascular disease in OSAHS: the oxidative stress link. Eur Respir J. 2009;33:1467–84.
Victor VM, Rocha M, Sola E, Banuls C, Garcia-Malpartida K, Hernandez-Mijares A. Oxidative stress, endothelial dysfunction and atherosclerosis. Curr Pharm Des. 2009;15:2988–3002.
Lavie L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia–revisited–the bad ugly and good: implications to the heart and brain. Sleep Med Rev. 2015;20:27–45.
Mansukhani MP, Kara T, Caples SM, Somers VK. Chemoreflexes, sleep apnea, and sympathetic dysregulation. Curr Hypertens Rep. 2014;16:476.
Cooper VL, Bowker CM, Pearson SB, Elliott MW, Hainsworth R. Effects of simulated obstructive sleep apnoea on the human carotid baroreceptor-vascular resistance reflex. J Physiol. 2004;557:1055–65.
Ziegler MG, Milic M, Elayan H. Cardiovascular regulation in obstructive sleep apnea. Drug Discov Today Dis models. 2011;8:155–60.
Narkiewicz K, van de Borne PJ, Cooley RL, Dyken ME, Somers VK. Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation. 1998;98:772–6.
Linz D, Mahfoud F, Schotten U, Ukena C, Neuberger HR, Wirth K, et al. Renal sympathetic denervation suppresses postapneic blood pressure rises and atrial fibrillation in a model for sleep apnea. Hypertension. 2012;60:172–8.
Rossi E, Sani C, Perazzoli F, Casoli MC, Negro A, Dotti C. Alterations of calcium metabolism and of parathyroid function in primary aldosteronism, and their reversal by spironolactone or by surgical removal of aldosterone-producing adenomas. Am J Hypertens. 1995;8:884–93.
Pilz S, Kienreich K, Drechsler C, Ritz E, Fahrleitner-Pammer A, Gaksch M, et al. Hyperparathyroidism in patients with primary aldosteronism: cross-sectional and interventional data from the GECOH study. J Clin Endocrinol Metab. 2012;97:E75–9.
Loh HH, Kamaruddin NA, Zakaria R, Sukor N. Improvement of bone turnover markers and bone mineral density following treatment of primary aldosteronism. Minerva Endocrinol. 2018;43:117–25.
Ceccoli L, Ronconi V, Giovannini L, Marcheggiani M, Turchi F, Boscaro M, et al. Bone health and aldosterone excess. Osteoporos Int. 2013;24:2801–7.
Petramala L, Zinnamosca L, Settevendemmie A, Marinelli C, Nardi M, Concistre A, et al. Bone and mineral metabolism in patients with primary aldosteronism. Int J Endocrinol. 2014;2014:836529.
Jiang Y, Zhang C, Ye L, Su T, Zhou W, Jiang L, et al. Factors affecting parathyroid hormone levels in different types of primary aldosteronism. Clin Endocrinol. 2016;85:267–74.
Salcuni AS, Palmieri S, Carnevale V, Morelli V, Battista C, Guarnieri V, et al. Bone involvement in aldosteronism. J Bone Miner Res. 2012;27:2217–22.
Loh HH, Yee A, Loh HS. Bone health among patients with primary aldosteronism: a systematic review and meta-analysis. Minerva Endocrinol. 2018. https://doi.org/10.23736/S0391-1977.18.02867-5.
Notsu M, Yamauchi M, Yamamoto M, Nawata K, Sugimoto T. Primary aldosteronism as a risk factor for vertebral fracture. J Clin Endocrinol Metab. 2017;102:1237–43.
Wu VC, Chang CH, Wang CY, Lin YH, Kao TW, Lin PC, et al. Risk of fracture in primary aldosteronism: a Population-Based Cohort Study. J Bone Miner Res. 2017;32:743–52.
Vasikaran S, Eastell R, Bruyere O, Foldes AJ, Garnero P, Griesmacher A, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22:391–420.
Carbone LD, Cross JD, Raza SH, Bush AJ, Sepanski RJ, Dhawan S, et al. Fracture risk in men with congestive heart failure risk reduction with spironolactone. J Am Coll Cardiol. 2008;52:135–8.
Brunaud L, Germain A, Zarnegar R, Rancier M, Alrasheedi S, Caillard C, et al. Serum aldosterone is correlated positively to parathyroid hormone (PTH) levels in patients with primary hyperparathyroidism. Surgery. 2009;146:1035–41.
Kovacs KA, Gay JD. Remission of primary hyperparathyroidism due to spontaneous infarction of a parathyroid adenoma. Case report and review of the literature. Medicine. 1998;77:398–402.
Pacifici R, Perry HM 3rd, Shieber W, Biglieri E, Droke DM, Avioli LV. Adrenal responses to subtotal parathyroidectomy for primary hyperparathyroidism. Calcif Tissue Int. 1987;41:119–23.
Bernini G, Moretti A, Lonzi S, Bendinelli C, Miccoli P, Salvetti A. Renin-angiotensin-aldosterone system in primary hyperparathyroidism before and after surgery. Metabolism. 1999;48:298–300.
Salahudeen AK, Thomas TH, Sellars L, Tapster S, Keavey P, Farndon JR, et al. Hypertension and renal dysfunction in primary hyperparathyroidism: effect of parathyroidectomy. Clin Sci. 1989;76:289–96.
Vaidya A, Brown JM, Williams JS. The renin-angiotensin-aldosterone system and calcium-regulatory hormones. J Hum Hypertens. 2015;29:515–21.
Fischer E, Hannemann A, Rettig R, Lieb W, Nauck M, Pallauf A, et al. A high aldosterone to renin ratio is associated with high serum parathyroid hormone concentrations in the general population. J Clin Endocrinol Metab. 2014;99:965–71.
Brown J, de Boer IH, Robinson-Cohen C, Siscovick DS, Kestenbaum B, Allison M, et al. Aldosterone, parathyroid hormone, and the use of renin-angiotensin-aldosterone system inhibitors: the multi-ethnic study of atherosclerosis. J Clin Endocrinol Metab. 2015;100:490–9.
Grant FD, Mandel SJ, Brown EM, Williams GH, Seely EW. Interrelationships between the renin-angiotensin-aldosterone and calcium homeostatic systems. J Clin Endocrinol Metab. 1992;75:988–92.
Brown JM, Williams JS, Luther JM, Garg R, Garza AE, Pojoga LH, et al. Human interventions to characterize novel relationships between the renin-angiotensin-aldosterone system and parathyroid hormone. Hypertension. 2014;63:273–80.
Maniero C, Fassina A, Guzzardo V, Lenzini L, Amadori G, Pelizzo MR, et al. Primary hyperparathyroidism with concurrent primary aldosteronism. Hypertension. 2011;58:341–6.
Hulter HN, Melby JC, Peterson JC, Cooke CR. Chronic continuous PTH infusion results in hypertension in normal subjects. J Clin Hypertens. 1986;2:360–70.
Mazzocchi G, Aragona F, Malendowicz LK, Nussdorfer GG. PTH and PTH-related peptide enhance steroid secretion from human adrenocortical cells. Am J Physiol Endocrinol Metab. 2001;280:E209–13.
Brown NJ. Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nat Rev Nephrol. 2013;9:459–69.
Armanini D, Andrisani A, Ambrosini G, Dona G, Camozzi V, Bordin L, et al. Interrelationship between vitamin D insufficiency, calcium homeostasis, hyperaldosteronism, and autoimmunity. J Clin Hypertens. 2016;18:614–6.
Pilon C, Urbanet R, Williams TA, Maekawa T, Vettore S, Sirianni R, et al. 1alpha,25-Dihydroxyvitamin D(3) inhibits the human H295R cell proliferation by cell cycle arrest: a model for a protective role of vitamin D receptor against adrenocortical cancer. J Steroid Biochem Mol Biol. 2014;140:26–33.
Lundqvist J, Wikvall K, Norlin M. Vitamin D-mediated regulation of CYP21A2 transcription—a novel mechanism for vitamin D action. Biochim Biophys Acta. 2012;1820:1553–9.
Tirabassi G, Salvio G, Altieri B, Ronchi CL, Della Casa S, Pontecorvi A, et al. Adrenal disorders: is there any role for vitamin D? Rev Endocr Metab Disord. 2017;18:355–62.
Author information
Authors and Affiliations
Contributions
HHL conceived and designed the work that led to the submission, acquired data, and interpreted the results. HHL drafted the manuscript while NS revised the manuscript. Both authors approved the final version and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Loh, H.H., Sukor, N. Associations between primary aldosteronism and diabetes, poor bone health, and sleep apnea—what do we know so far?. J Hum Hypertens 34, 5–15 (2020). https://doi.org/10.1038/s41371-019-0294-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41371-019-0294-8
This article is cited by
-
Associations Between Aldosterone-Renin-Ratio and Bone Parameters Derived from Peripheral Quantitative Computed Tomography and Impact Microindentation in Men
Calcified Tissue International (2023)
-
Epidemiology and diagnosis of primary aldosteronism. What have we learned from the SPAIN-ALDO registry?
Endocrine (2023)
-
Recovery from diabetes mellitus in primary aldosteronism patients after adrenalectomy
BMC Endocrine Disorders (2022)