Abstract
Objective
Fatty acids play a critical role in the proper functioning of the brain. This study investigated the effects of a high-fat (HF) diet on brain fatty acid profiles of offspring exposed to maternal gestational diabetes mellitus (GDM).
Methods
Insulin receptor antagonist (S961) and HF diet were used to establish the GDM animal model. Brain fatty acid profiles of the offspring mice were measured by gas chromatography at weaning and adulthood. Protein expressions of the fatty acid transport pathway Wnt3/β-catenin and the target protein major facilitator superfamily domain-containing 2a (MFSD2a) were measured in the offspring brain by Western blot.
Results
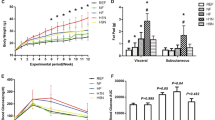
Maternal GDM increased the body weight of male offspring (P < 0.05). In weaning offspring, factorial analysis showed that maternal GDM increased the monounsaturated fatty acid (MUFA) percentage of the weaning offspring’s brain (P < 0.05). Maternal GDM decreased offspring brain arachidonic acid (AA), but HF diet increased brain linoleic acid (LA) (P < 0.05). Maternal GDM and HF diet reduced offspring brain docosahexaenoic acid (DHA), and the male offspring had higher DHA than the female offspring (P < 0.05). In adult offspring, factorial analysis showed that HF diet increased brain MUFA in offspring, and male offspring had higher brain MUFA than female offspring (P < 0.05). The HF diet increased brain LA in the offspring. Male offspring had higher level of AA than female offspring (P < 0.05). HF diet reduced DHA in the brains of female offspring. The brain protein expression of β-catenin and MFSD2a in both weaning and adult female offspring was lower in the HF + GDM group than in the CON group (P < 0.05).
Conclusions
Maternal GDM increased the susceptibility of male offspring to HF diet-induced obesity. HF diet-induced adverse brain fatty acid profiles in both male and female offspring exposed to GDM.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available in the Mendeley Data repository [https://doi.org/10.17632/725p5xz7yx.1].
References
Zeng J, Shen F, Zou ZY, Yang RX, Jin Q, Yang J, et al. Association of maternal obesity and gestational diabetes mellitus with overweight/obesity and fatty liver risk in offspring. World J Gastroenterol. 2022;28:1681–91.
Nielsen GL, Andersen E, Lundbye-Christensen S. Maternal blood glucose in diabetic pregnancies and cognitive performance in offspring in young adulthood: a Danish cohort study. Diabet Med. 2010;27:786–90.
Nomura Y, Marks DJ, Grossman B, Yoon M, Loudon H, Stone J, et al. Exposure to gestational diabetes mellitus and low socioeconomic status: effects on neurocognitive development and risk of attention-deficit/hyperactivity disorder in offspring. Arch Pediatr Adolesc Med. 2012;166:337–43.
Moore TR. Fetal exposure to gestational diabetes contributes to subsequent adult metabolic syndrome. Am J Obstet Gynecol. 2010;202:643–9.
Zornoza-Moreno M, Fuentes-Hernandez S, Carrion V, Alcantara-Lopez MV, Madrid JA, Lopez-Soler C, et al. Is low docosahexaenoic acid associated with disturbed rhythms and neurodevelopment in offsprings of diabetic mothers? Eur J Clin Nutr. 2014;68:931–7.
Dionne G, Boivin M, Séguin JR, Pérusse D, Tremblay RE. Gestational diabetes hinders language development in offspring. Pediatrics. 2008;122:e1073–9.
Fraser A, Nelson SM, Macdonald-Wallis C, Lawlor DA. Associations of existing diabetes, gestational diabetes, and glycosuria with offspring IQ and educational attainment: the Avon Longitudinal Study of Parents and Children. Exp Diabetes Res. 2012;2012:963735.
Bersain R, Mishra D, Juneja M, Kumar D, Garg S. Comparison of neurodevelopmental status in early infancy of infants of women with and without gestational diabetes mellitus. Indian J Pediatr. 2023;90:1083–8.
He XJ, Dai RX, Tian CQ, Hu CL. Neurodevelopmental outcome at 1 year in offspring of women with gestational diabetes mellitus. Gynecol Endocrinol. 2021;37:88–92.
Munoz-Islas E, Elizondo-Martinez CE, Gutierrez-Lopez M, Acosta-Gonzalez RI, Zaga-Clavellina V, Helguera-Repetto AC, et al. Effect of experimental gestational diabetes mellitus on mechanical sensitivity, capsaicin-induced pain behaviors and hind paw glabrous skin innervation of male and female mouse offspring. J Pain Res. 2021;14:1573–85.
Le Moullec N, Fianu A, Maillard O, Chazelle E, Naty N, Schneebeli C, et al. Sexual dimorphism in the association between gestational diabetes mellitus and overweight in offspring at 5-7 years: The OBEGEST Cohort Study. PLoS One. 2018;13:e0195531.
Prieto-Sanchez MT, Ruiz-Palacios M, Blanco-Carnero JE, Pagan A, Hellmuth C, Uhl O, et al. Placental MFSD2a transporter is related to decreased DHA in cord blood of women with treated gestational diabetes. Clin Nutr. 2017;36:513–21.
Nguyen LN, Ma D, Shui G, Wong P, Cazenave-Gassiot A, Zhang X, et al. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 2014;509:503–6.
Wang ZX, Liu CH, Huang S, Fu ZJ, Tomita Y, Britton WR, et al. Wnt signaling activates MFSD2A to suppress vascular endothelial transcytosis and maintain blood-retinal barrier. Sci Adv. 2020;6:eaba7457.
Pagán A, Prieto-Sánchez MT, Blanco-Carnero JE, Gil-Sánchez A, Parrilla JJ, Demmelmair H, et al. Materno-fetal transfer of docosahexaenoic acid is impaired by gestational diabetes mellitus. Am J Physiol Endocrinol Metab. 2013;305:E826–33.
Regnault N, Gillman MW, Rifas-Shiman SL, Eggleston E, Oken E. Sex-specific associations of gestational glucose tolerance with childhood body composition. Diabetes Care. 2013;36:3045–53.
Du Q, Sompolinsky Y, Walfisch A, Zhong H, Liu Y, Feng W. The sex specific association between maternal gestational diabetes and offspring metabolic status at 1 year of age. Front Endocrinol. 2020;11:608125.
Yu H-T, Gong J-Y, Xu W-H, Chen Y-R, Li Y-T, Chen Y-F, et al. Gestational diabetes mellitus remodels the fetal brain fatty acid profile through placenta-brain lipid axis in C57BL/6J mice. J Nutr. 2023; https://doi.org/10.1016/j.tjnut.2023.12.045.
Xu WH, Gong JY, Chen YF, Chen YR, Chen ST, Wu YY, et al. Effects of gestational diabetes mellitus and selenium deficiency on the offspring growth and blood glucose mechanisms of C57BL/6J mice. Nutrients. 2023;15:4519.
Pulgaron ER, Delamater AM. Obesity and type 2 diabetes in children: epidemiology and treatment. Curr Diabetes Rep. 2014;14:508.
Kawasaki M, Arata N, Miyazaki C, Mori R, Kikuchi T, Ogawa Y, et al. Obesity and abnormal glucose tolerance in offspring of diabetic mothers: A systematic review and meta-analysis. PLoS One. 2018;13:e0190676.
Vandyousefi S, Whaley SE, Widen EM, Asigbee FM, Landry MJ, Ghaddar R, et al. Association of breastfeeding and early exposure to sugar-sweetened beverages with obesity prevalence in offspring born to mothers with and without gestational diabetes mellitus. Pediatr Obes. 2019;14:e12569.
Grunnet LG, Hansen S, Hjort L, Madsen CM, Kampmann FB, Thuesen ACB, et al. Adiposity, dysmetabolic traits, and earlier onset of female puberty in adolescent offspring of women with gestational diabetes mellitus: a clinical study within the danish national birth cohort. Diabetes Care. 2017;40:1746–55.
Shapiro ALB, Sauder KA, Tregellas JR, Legget KT, Gravitz SL, Ringham BM, et al. Exposure to maternal diabetes in utero and offspring eating behavior: The EPOCH study. Appetite. 2017;116:610–5.
Kumar D, Shankar K, Patel S, Gupta A, Varshney S, Gupta S, et al. Chronic hyperinsulinemia promotes meta-inflammation and extracellular matrix deposition in adipose tissue: Implications of nitric oxide. Mol Cell Endocrinol. 2018;477:15–28.
Mehran AryaE, Templeman NicoleM, Brigidi GS, Lim GarethE, Chu K-Y, Hu X, et al. Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metabol. 2012;16:723–37.
Bendor CD, Bardugo A, Rotem RS, Derazne E, Gerstein HC, Tzur D, et al. Glucose intolerance in pregnancy and offspring obesity in late adolescence. Diabetes Care. 2022;45:1540–8.
Tsadok MA, Friedlander Y, Paltiel O, Manor O, Meiner V, Hochner H, et al. Obesity and blood pressure in 17-year-old offspring of mothers with gestational diabetes: insights from the jerusalem perinatal study. Exp Diabetes Res. 2011;2011:906154.
Krishnaveni GV, Veena SR, Hill JC, Kehoe S, Karat SC, Fall CH. Intrauterine exposure to maternal diabetes is associated with higher adiposity and insulin resistance and clustering of cardiovascular risk markers in Indian children. Diabetes Care. 2010;33:402–4.
Hu J, Liu Y, Wei X, Li L, Gao M, Liu Y, et al. Association of gestational diabetes mellitus with offspring weight status across infancy: a prospective birth cohort study in China. BMC Pregnancy Childbirth. 2021;21:21.
Li S, Zhu Y, Yeung E, Chavarro JE, Yuan C, Field AE, et al. Offspring risk of obesity in childhood, adolescence and adulthood in relation to gestational diabetes mellitus: a sex-specific association. Int J Epidemiol. 2017;46:1533–41.
Oraha J, Enriquez RF, Herzog H, Lee NJ. Sex-specific changes in metabolism during the transition from chow to high-fat diet feeding are abolished in response to dieting in C57BL/6J mice. Int J Obes. 2022;46:1749–58.
Palmisano BT, Stafford JM, Pendergast JS. High-fat feeding does not disrupt daily rhythms in female mice because of protection by ovarian hormones. Front Endocrinol. 2017;8:44.
Lau SM, Lin S, Stokes RA, Cheng K, Baldock PA, Enriquez RF, et al. Synergistic effects of genetic beta cell dysfunction and maternal glucose intolerance on offspring metabolic phenotype in mice. Diabetologia. 2011;54:910–21.
Xiang AH, Wang X, Martinez MP, Walthall JC, Curry ES, Page K, et al. Association of maternal diabetes with autism in offspring. Jama. 2015;313:1425–34.
Krakowiak P, Walker CK, Bremer AA, Baker AS, Ozonoff S, Hansen RL, et al. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics. 2012;129:E1121–E8.
Chandna AR, Kuhlmann N, Bryce CA, Greba Q, Campanucci VA, Howland JG. Chronic maternal hyperglycemia induced during mid-pregnancy in rats increases RAGE expression, augments hippocampal excitability, and alters behavior of the offspring. Neuroscience. 2015;303:241–60.
Golalipour MJ, Kafshgiri SK, Ghafari S. Gestational diabetes induced neuronal loss in CA1 and CA3 subfields of rat hippocampus in early postnatal life. Folia Morphologica. 2012;71:71–7.
Ogundipe E, Samuelson S, Crawford MA. Gestational diabetes mellitus prediction? A unique fatty acid profile study. Nutr Diabetes. 2020;10:36.
Saben JL, Bales ES, Jackman MR, Orlicky D, MacLean PS, McManaman JL. Maternal obesity reduces milk lipid production in lactating mice by inhibiting acetyl-CoA carboxylase and impairing fatty acid synthesis. PLoS One. 2014;9:e98066.
Wen L, Wu Y, Yang Y, Han T-L, Wang W, Fu H, et al. Gestational diabetes mellitus changes the metabolomes of human colostrum, transition milk and mature milk. Med Sci Monitor. 2019;25:6128–52.
Rodríguez-Cruz M, Sánchez R, Bernabe-Garcia M, Maldonado J, Del Prado M, López-Alarcón M. Effect of dietary levels of corn oil on maternal arachidonic acid synthesis and fatty acid composition in lactating rats. Nutrition. 2009;25:209–15.
Nasser R, Stephen AM, Goh YK, Clandinin MT. The effect of a controlled manipulation of maternal dietary fat intake on medium and long chain fatty acids in human breast milk in Saskatoon, Canada. Int Breastfeeding J. 2010;5:3.
Author information
Authors and Affiliations
Contributions
HT Yu conducted the study, analyzed the data and wrote the article;WH Xu, JY Gong and YF Chen feed the animal; Y He, ST Chen, YY Wu measured the fatty acids; GL Liu, HY Zhang: provided essential reagents and materials; L Xie designed the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, HT., Xu, WH., Gong, JY. et al. Effect of high-fat diet on the fatty acid profiles of brain in offspring mice exposed to maternal gestational diabetes mellitus. Int J Obes (2024). https://doi.org/10.1038/s41366-024-01486-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41366-024-01486-7